Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene
- PMID: 19101982
- PMCID: PMC2657345
- DOI: 10.1002/hep.22695
Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene
Abstract
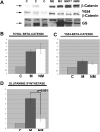
Wnt/beta-catenin signaling plays an important role in liver development and regeneration. Its aberrant activation, however, is observed in a subset of primary hepatocellular cancers (HCCs). In the current study, we compare and contrast the tumor characteristics of HCC in the presence or absence of mutations in the beta-catenin gene (CTNNB1). Frozen HCCs (n = 32), including five fibrolamellar (FL) variants, and control livers (n = 3) from Health Sciences Tissue Bank and Department of Surgery at the University of Pittsburgh Medical Center, were examined for mutations in CTNNB1, protein levels of beta-catenin, tyrosine-654-phosphorylated-beta-catenin (Y654-beta-catenin), and glutamine synthetase (GS). Missense mutations in the exon-3 of CTNNB1were identified in 9/32 HCCs. Total beta-catenin levels were higher than controls in most tumors; however, GS was exclusively increased in HCCs with mutations. Phenotypically, greater percentages of mutated HCCs showed macrovascular and microvascular invasion. Also, the tumor size was greater than double in mutated HCCs. High levels of total beta-catenin protein were observed in multinodular tumors independent of beta-catenin mutations. In addition, significant cases with mutations showed absence of cirrhosis. Finally, the highest levels of Y654-beta-catenin were exclusively observed in fibrolamellar (FL)-HCC cases.
Conclusion: Thus, HCCs that harbor missense mutations in exon-3 of CTNNB1 exhibit, histologically, a more aggressive phenotype. Also, CTNNB1 mutations might lead to HCC in the absence of cirrhosis. Finally, FL-HCC cases display a unique up-regulation of tyrosine-phosphorylated-beta-catenin, suggesting robust receptor tyrosine kinase signaling in this tumor type.
Figures






References
-
- El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Bergsland EK, Venook AP. Hepatocellular carcinoma. Curr Opin Oncol. 2000;12:357–361. - PubMed
-
- McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006;136:125–135. - PubMed
-
- Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
