The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913)
- PMID: 19102734
- PMCID: PMC2637262
- DOI: 10.1186/1471-244X-8-95
The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913)
Abstract
Background: The aim of this paper is to evaluate the effect of antipsychotics for the treatment of schizophrenia in a community based study on sexual function and prolactin levels comparing the use of aripiprazole and standard of care (SOC), which was a limited choice of three widely used and available antipsychotics (olanzapine, quetiapine or risperidone) (The Schizophrenia Trial of Aripiprazole [STAR] study [NCT00237913]).
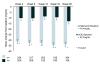
Method: This open-label, 26-week, multi-centre, randomised study compared aripiprazole to SOC (olanzapine, quetiapine or risperidone) in patients with schizophrenia (DSM-IV-TR criteria). The primary effectiveness variable was the mean total score of the Investigator Assessment Questionnaire (IAQ) at Week 26. The outcome research variables included the Arizona Sexual Experience scale (ASEX). This along with the data collected on serum prolactin levels at week 4, 8, 12, 18 and 26 will be the focus of this paper.
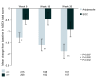
Results: A total of 555 patients were randomised to receive aripiprazole (n = 284) or SOC (n = 271). Both treatment groups experienced improvements in sexual function from baseline ASEX assessments. However at 8 weeks the aripiprazole treatment group reported significantly greater improvement compared with the SOC group (p = 0.007; OC). Although baseline mean serum prolactin levels were similar in the two treatment groups (43.4 mg/dL in the aripiprazole group and 42.3 mg/dL in the SOC group, p = NS) at Week 26 OC, mean decreases in serum prolactin were 34.2 mg/dL in the aripiprazole group, compared with 13.3 mg/dL in the SOC group (p < 0.001).
Conclusion: The study findings suggest that aripiprazole has the potential to reduce sexual dysfunction, which in turn might improve patient compliance.
Figures
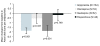

Similar articles
-
A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study.Eur Psychiatry. 2007 Oct;22(7):433-43. doi: 10.1016/j.eurpsy.2007.03.002. Epub 2007 Jun 7. Eur Psychiatry. 2007. PMID: 17555947 Clinical Trial.
-
Changes in weight and weight-related quality of life in a multicentre, randomized trial of aripiprazole versus standard of care.Eur Psychiatry. 2008 Dec;23(8):561-6. doi: 10.1016/j.eurpsy.2008.01.1421. Epub 2008 Apr 18. Eur Psychiatry. 2008. PMID: 18374544 Clinical Trial.
-
A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP).Am J Psychiatry. 2011 Sep;168(9):947-56. doi: 10.1176/appi.ajp.2011.10111609. Epub 2011 Jul 18. Am J Psychiatry. 2011. PMID: 21768610 Free PMC article. Clinical Trial.
-
An open, large, 6-month naturalistic study of outcome in schizophrenic outpatients, treated with olanzapine.Hum Psychopharmacol. 2011 Jan;26(1):81-5. doi: 10.1002/hup.1173. Epub 2011 Feb 9. Hum Psychopharmacol. 2011. PMID: 23055416 Review.
-
Efficacy and safety of atypical antipsychotic drugs (quetiapine, risperidone, aripiprazole and paliperidone) compared with placebo or typical antipsychotic drugs for treating refractory schizophrenia: overview of systematic reviews.Sao Paulo Med J. 2010 May;128(3):141-66. doi: 10.1590/s1516-31802010000300007. Sao Paulo Med J. 2010. PMID: 20963366 Free PMC article. Review.
Cited by
-
Olanzapine induced reproductive toxicity in male rats.Sci Rep. 2021 Feb 26;11(1):4739. doi: 10.1038/s41598-021-84235-4. Sci Rep. 2021. PMID: 33637793 Free PMC article.
-
Reduced sexual dysfunction with aripiprazole once-monthly versus paliperidone palmitate: results from QUALIFY.Int Clin Psychopharmacol. 2017 May;32(3):147-154. doi: 10.1097/YIC.0000000000000168. Int Clin Psychopharmacol. 2017. PMID: 28252452 Free PMC article. Clinical Trial.
-
Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care.World Psychiatry. 2011 Feb;10(1):52-77. doi: 10.1002/j.2051-5545.2011.tb00014.x. World Psychiatry. 2011. PMID: 21379357 Free PMC article.
-
Sexual dysfunction in male subjects receiving trifluoperazine, risperidone, or olanzapine: rates vary with assessment questionnaire.Prim Care Companion CNS Disord. 2012;14(2):PCC.11m01199. doi: 10.4088/PCC.11m01199. Epub 2012 Apr 26. Prim Care Companion CNS Disord. 2012. PMID: 22943029 Free PMC article.
-
Serum prolactin levels and sexual dysfunction in patients with schizophrenia treated with antipsychotics: comparison between aripiprazole and other atypical antipsychotics.Ann Gen Psychiatry. 2017 Nov 28;16:43. doi: 10.1186/s12991-017-0166-y. eCollection 2017. Ann Gen Psychiatry. 2017. PMID: 29209406 Free PMC article.
References
-
- Klibanski A, Neer RM, Beitins IZ, et al. Decreased bone density in hyperprolactinemic women. N Engl J Med. 1980;303:1511–1514. - PubMed
-
- Smith S, Gillam A. Sexual dysfunction – the forgotten taboo. Mental Health Nurs. 2005;25:6–9.
-
- Gopalakrishnan R, Jacob KS, Kuruvilla A, Vasantharaj B, John JK. Sildenafil in the Treatment of Antipsychotic-Induced Erectile Dysfunction: A Randomized, Double-Blind, Placebo-Controlled, Flexible-Dose, Two-Way Crossover Trial. Am J Psychiatry. 2006;163:494–499. doi: 10.1176/appi.ajp.163.3.494. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

