A randomized, open-label, comparative efficacy trial of artemether-lumefantrine suspension versus artemether-lumefantrine tablets for treatment of uncomplicated Plasmodium falciparum malaria in children in western Kenya
- PMID: 19102746
- PMCID: PMC2635380
- DOI: 10.1186/1475-2875-7-262
A randomized, open-label, comparative efficacy trial of artemether-lumefantrine suspension versus artemether-lumefantrine tablets for treatment of uncomplicated Plasmodium falciparum malaria in children in western Kenya
Abstract
Background: Artemether/lumefantrine (AL) has been adopted as the treatment of choice for uncomplicated malaria in Kenya and other countries in the region. Six-dose artemether/lumefantrine tablets are highly effective and safe for the treatment of infants and children weighing between five and 25 kg with uncomplicated Plasmodium falciparum malaria. However, oral paediatric formulations are urgently needed, as the tablets are difficult to administer to young children, who cannot swallow whole tablets or tolerate the bitter taste of the crushed tablets.
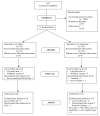
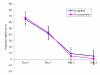
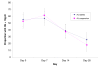
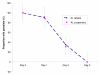
Methods: A randomized, controlled, open-label trial was conducted comparing day 28 PCR corrected cure-rates in 245 children aged 6-59 months, treated over three days with either six-dose of artemether/lumefantrine tablets (Coartem) or three-dose of artemether/lumefantrine suspension (Co-artesiane) for uncomplicated falciparum malaria in western Kenya. The children were followed-up with clinical, parasitological and haematological evaluations over 28 days.
Results: Ninety three percent (124/133) and 90% (121/134) children in the AL tablets and AL suspension arms respectively completed followed up. A per protocol analysis revealed a PCR-corrected parasitological cure rate of 96.0% at Day 28 in the AL tablets group and 93.4% in the AL suspension group, p = 0.40. Both drugs effectively cleared gametocytes and were well tolerated, with no difference in the overall incidence of adverse events.
Conclusion: The once daily three-dose of artemether-lumefantrine suspension (Co-artesiane(R)) was not superior to six-dose artemether-lumefantrine tablets (Coartem) for the treatment of uncomplicated malaria in children below five years of age in western Kenya.
Trial registration: ClinicalTrials.gov NCT00529867.
Figures
Similar articles
-
Dispersible formulation of artemether/lumefantrine: specifically developed for infants and young children.Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S7. doi: 10.1186/1475-2875-8-S1-S7. Malar J. 2009. PMID: 19818174 Free PMC article. Review.
-
Therapeutic efficacy of artemether-lumefantrine in uncomplicated falciparum malaria in India.Malar J. 2009 May 19;8:107. doi: 10.1186/1475-2875-8-107. Malar J. 2009. PMID: 19454000 Free PMC article. Clinical Trial.
-
Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study.Malar J. 2014 Jan 28;13:33. doi: 10.1186/1475-2875-13-33. Malar J. 2014. PMID: 24472156 Free PMC article. Clinical Trial.
-
Efficacy and safety of a fixed dose artesunate-sulphamethoxypyrazine-pyrimethamine compared to artemether-lumefantrine for the treatment of uncomplicated falciparum malaria across Africa: a randomized multi-centre trial.Malar J. 2009 Apr 14;8:63. doi: 10.1186/1475-2875-8-63. Malar J. 2009. PMID: 19366448 Free PMC article. Clinical Trial.
-
Paediatric formulations of artemisinin-based combination therapies for treating uncomplicated malaria in children.Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD009568. doi: 10.1002/14651858.CD009568.pub2. Cochrane Database Syst Rev. 2020. PMID: 33289099 Free PMC article.
Cited by
-
New perspectives on chinese herbal medicine (zhong-yao) research and development.Evid Based Complement Alternat Med. 2011;2011:403709. doi: 10.1093/ecam/neq056. Epub 2011 Mar 10. Evid Based Complement Alternat Med. 2011. PMID: 21785622 Free PMC article.
-
Dispersible formulation of artemether/lumefantrine: specifically developed for infants and young children.Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S7. doi: 10.1186/1475-2875-8-S1-S7. Malar J. 2009. PMID: 19818174 Free PMC article. Review.
-
A randomized trial of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated malaria among children in western Kenya.Malar J. 2013 Jul 19;12:254. doi: 10.1186/1475-2875-12-254. Malar J. 2013. PMID: 23870627 Free PMC article. Clinical Trial.
-
Current status of the clinical development and implementation of paediatric artemisinin combination therapies in Sub-Saharan Africa.Wien Klin Wochenschr. 2011 Oct;123 Suppl 1:7-9. doi: 10.1007/s00508-011-0039-3. Epub 2011 Aug 6. Wien Klin Wochenschr. 2011. PMID: 21826416
-
Changing malaria intervention coverage, transmission and hospitalization in Kenya.Malar J. 2010 Oct 15;9:285. doi: 10.1186/1475-2875-9-285. Malar J. 2010. PMID: 20946689 Free PMC article.
References
-
- Zucker JR, Ruebush TK, Obonyo C, Otieno J, Campbell CC. The mortality consequences of the continued use of chloroquine in Africa: experience in Siaya, western Kenya. Am J Trop Med Hyg. 2003;68:386–390. - PubMed
-
- Falade C, Makanga M, Premji Z, Ortmann CE, Stockmeyer M, Ibarra de Palacios P. Efficacy and safety of artemether-lumefantrine (Coartem®) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99:459–467. doi: 10.1016/j.trstmh.2004.09.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical