Evidence and Value: Impact on DEcisionMaking--the EVIDEM framework and potential applications
- PMID: 19102752
- PMCID: PMC2673218
- DOI: 10.1186/1472-6963-8-270
Evidence and Value: Impact on DEcisionMaking--the EVIDEM framework and potential applications
Abstract
Background: Healthcare decisionmaking is a complex process relying on disparate types of evidence and value judgments. Our objectives for this study were to develop a practical framework to facilitate decisionmaking in terms of supporting the deliberative process, providing access to evidence, and enhancing the communication of decisions.
Methods: Extensive analyses of the literature and of documented decisionmaking processes around the globe were performed to explore what steps are currently used to make decisions with respect to context (from evidence generation to communication of decision) and thought process (conceptual components of decisions). Needs and methodologies available to support decisionmaking were identified to lay the groundwork for the EVIDEM framework.
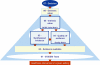
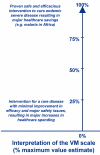
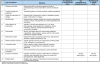
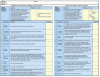
Results: A framework was developed consisting of seven modules that can evolve over the life cycle of a healthcare intervention. Components of decision that could be quantified, i.e., intrinsic value of a healthcare intervention and quality of evidence available, were organized into matrices. A multicriteria decision analysis (MCDA) Value Matrix (VM) was developed to include the 15 quantifiable components that are currently considered in decisionmaking. A methodology to synthesize the evidence needed for each component of the VM was developed including electronic access to full text source documents. A Quality Matrix was designed to quantify three criteria of quality for the 12 types of evidence usually required by decisionmakers. An integrated system was developed to optimize data analysis, synthesis and validation by experts, compatible with a collaborative structure.
Conclusion: The EVIDEM framework promotes transparent and efficient healthcare decisionmaking through systematic assessment and dissemination of the evidence and values on which decisions are based. It provides a collaborative framework that could connect all stakeholders and serve the healthcare community at local, national and international levels by allowing sharing of data, resources and values. Validation and further development is needed to explore the full potential of this approach.
Figures




Similar articles
-
Bridging health technology assessment (HTA) with multicriteria decision analyses (MCDA): field testing of the EVIDEM framework for coverage decisions by a public payer in Canada.BMC Health Serv Res. 2011 Nov 30;11:329. doi: 10.1186/1472-6963-11-329. BMC Health Serv Res. 2011. PMID: 22129247 Free PMC article.
-
Field testing of a multicriteria decision analysis (MCDA) framework for coverage of a screening test for cervical cancer in South Africa.Cost Eff Resour Alloc. 2012 Feb 29;10(1):2. doi: 10.1186/1478-7547-10-2. Cost Eff Resour Alloc. 2012. PMID: 22376143 Free PMC article.
-
Combining multicriteria decision analysis, ethics and health technology assessment: applying the EVIDEM decision-making framework to growth hormone for Turner syndrome patients.Cost Eff Resour Alloc. 2010 Apr 8;8:4. doi: 10.1186/1478-7547-8-4. Cost Eff Resour Alloc. 2010. PMID: 20377888 Free PMC article.
-
Can reflective multicriteria be the new paradigm for healthcare decision-making? The EVIDEM journey.Cost Eff Resour Alloc. 2018 Nov 9;16(Suppl 1):54. doi: 10.1186/s12962-018-0116-9. eCollection 2018. Cost Eff Resour Alloc. 2018. PMID: 30455613 Free PMC article. Review.
-
Identifying key unmet needs and value drivers in the treatment of focal-onset seizures (FOS) in patients with drug-resistant epilepsy (DRE) in Spain through Multi-Criteria Decision Analysis (MCDA).Epilepsy Behav. 2021 Sep;122:108222. doi: 10.1016/j.yebeh.2021.108222. Epub 2021 Aug 6. Epilepsy Behav. 2021. PMID: 34371462
Cited by
-
Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.Pharmacoeconomics. 2013 May;31(5):361-7. doi: 10.1007/s40273-013-0032-y. Pharmacoeconomics. 2013. PMID: 23529207
-
Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations.Pharmacoeconomics. 2022 Jun;40(6):601-609. doi: 10.1007/s40273-021-01112-8. Epub 2022 Jan 11. Pharmacoeconomics. 2022. PMID: 35015272 Free PMC article.
-
Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations.Health Policy Open. 2022 Jan 5;3:100063. doi: 10.1016/j.hpopen.2021.100063. eCollection 2022 Dec. Health Policy Open. 2022. PMID: 37383570 Free PMC article.
-
Multicriteria decision analysis (MCDA) in health care: a systematic review of the main characteristics and methodological steps.BMC Med Inform Decis Mak. 2018 Nov 1;18(1):90. doi: 10.1186/s12911-018-0663-1. BMC Med Inform Decis Mak. 2018. PMID: 30382826 Free PMC article.
-
Systematic review for the development of a pharmaceutical and medical products prioritization framework.J Pharm Policy Pract. 2019 Aug 21;12:21. doi: 10.1186/s40545-019-0181-2. eCollection 2019. J Pharm Policy Pract. 2019. PMID: 31452901 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

