Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication
- PMID: 19102781
- PMCID: PMC2635386
- DOI: 10.1186/1742-4690-5-117
Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication
Abstract
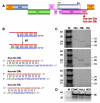
Background: Cellular miRNAs play an important role in the regulation of gene expression in eukaryotes. Recently, miRNAs have also been shown to be able to target and inhibit viral gene expression. Computational predictions revealed earlier that the HIV-1 genome includes regions that may be potentially targeted by human miRNAs. Here we report the functionality of predicted miR-29a target site in the HIV-1 nef gene.
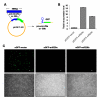
Results: We find that the human miRNAs hsa-miR-29a and 29b are expressed in human peripheral blood mononuclear cells. Expression of a luciferase reporter bearing the nef miR-29a target site was decreased compared to the luciferase construct without the target site. Locked nucleic acid modified anti-miRNAs targeted against hsa-miR-29a and 29b specifically reversed the inhibitory effect mediated by cellular miRNAs on the target site. Ectopic expression of the miRNA results in repression of the target Nef protein and reduction of virus levels.
Conclusion: Our results show that the cellular miRNA hsa-miR29a downregulates the expression of Nef protein and interferes with HIV-1 replication.
Figures


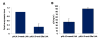
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

