A test of financial incentives to improve warfarin adherence
- PMID: 19102784
- PMCID: PMC2635367
- DOI: 10.1186/1472-6963-8-272
A test of financial incentives to improve warfarin adherence
Abstract
Background: Sub-optimal adherence to warfarin places millions of patients at risk for stroke and bleeding complications each year. Novel methods are needed to improve adherence for warfarin. We conducted two pilot studies to determine whether a lottery-based daily financial incentive is feasible and improves warfarin adherence and anticoagulation control.
Methods: Volunteers from the University of Pennsylvania Anticoagulation Management Center who had taken warfarin for at least 3 months participated in either a pilot study with a lottery with a daily expected value of $5 (N = 10) or a daily expected value of $3 (N = 10). All subjects received use of an Informedix Med-eMonitor System with a daily reminder feature. If subjects opened up their pill compartments appropriately, they were entered into a daily lottery with a 1 in 5 chance of winning $10 and a 1 in 100 chance of winning $100 (pilot 1) or a 1 in 10 chance of winning $10 and a 1 in 100 chance of winning $100 (pilot 2). The primary study outcome was proportion of incorrect warfarin doses. The secondary outcome was proportion of INR measurements not within therapeutic range. Within-subject pre-post comparisons were done of INR measurements with comparisons with either historic means or within-subject comparisons of incorrect warfarin doses.
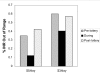
Results: In the first pilot, the percent of out-of-range INRs decreased from 35.0% to 12.2% during the intervention, before increasing to 42% post-intervention. The mean proportion of incorrect pills taken during the intervention was 2.3% incorrect pills, compared with a historic mean of 22% incorrect pill taking in this clinic population. Among the five subjects who also had MEMS cap adherence data from warfarin use in our prior study, mean incorrect pill taking decreased from 26% pre-pilot to 2.8% in the pilot. In the second pilot, the time out of INR range decreased from 65.0% to 40.4%, with the proportion of mean incorrect pill taking dropping to 1.6%.
Conclusion: A daily lottery-based financial incentive demonstrated the potential for significant improvements in missed doses of warfarin and time out of INR range. Further testing should be done of this approach to determine its effectiveness and potential application to both warfarin and other chronic medications.
Figures
References
-
- Madden KP, Karanjia PN, Adams HP, Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in Acute Stroke Treatment. Neurology. 1995;45:1975–1979. - PubMed
-
- Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA. 1998;279:1265–1272. doi: 10.1001/jama.279.16.1265. - DOI - PubMed
-
- Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous