Plasmodium falciparum secretory pathway: characterization of PfStx1, a plasma membrane Qa-SNARE
- PMID: 19103232
- PMCID: PMC2643330
- DOI: 10.1016/j.molbiopara.2008.11.011
Plasmodium falciparum secretory pathway: characterization of PfStx1, a plasma membrane Qa-SNARE
Abstract
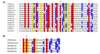
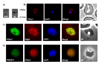
SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) play a central role in regulating and facilitating vesicular traffic in eukaryotic cells. While SNAREs have been well characterized in other eukaryotes, little is known about their role in the unique protein trafficking pathways in Plasmodium falciparum. We have identified seven Qa-SNAREs in the P. falciparum genome and confirmed the gene structure of all seven, which in one case differs from the predicted structure in the database. Based on comprehensive sequence alignments we made predictions for the intracellular locations of all seven P. falciparum Qa-SNAREs, and confirmed the predicted location for one Qa-SNARE, PfStx1, which is most closely related to other eukaryotic plasma membrane Qa-SNAREs such as syntaxin 1. This is the first identified trafficking component localized proximal to the P. falciparum plasma membrane.
Figures
References
-
- Struck NS, Herrmann S, Schmuck-Barkmann I, de Souza Dias S, Haase S, Cabrera AL, Treeck M, Bruns C, Langer C, Cowman AF, Marti M, Spielmann T, Gilberger TW. Spatial dissection of the cis- and trans-Golgi compartments in the malaria parasite Plasmodium falciparum. Mol Microbiol. 2008;67:1320–1330. - PubMed
-
- Yayon A, Timberg R, Friedman S, Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J Protozool. 1984;31:367–372. - PubMed
-
- Rudzinska MA, Trager W, Bray RS. Pinocytotic uptake and the digestion of hemoglobin in malaria parasites. J Protozool. 1965;12:563–576. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

