Mycoplasma genitalium-encoded MG309 activates NF-kappaB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells
- PMID: 19103762
- PMCID: PMC2643644
- DOI: 10.1128/IAI.00845-08
Mycoplasma genitalium-encoded MG309 activates NF-kappaB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells
Abstract
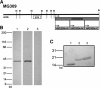
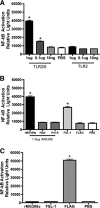
Mycoplasma genitalium has been implicated in several important reproductive tract syndromes in women, including pelvic inflammatory disease, cervicitis, and tubal factor infertility. The mechanisms of immune activation are unclear, and we sought to determine whether M. genitalium was capable of activating innate immune responses through ligation of highly expressed Toll-like receptors (TLR) of the genital tract. Using HEK293 cells expressing specific human TLR, viable M. genitalium and the recombinant C-terminal portion of the immunogenic protein MG309 (rMG309c) were shown to activate NF-kappaB via TLR2/6. These data provided a putative mechanism for activation of the innate response in genital tissues. Genital epithelial cells (EC) are the first responders to sexually transmitted pathogens and express high levels of TLR2 and -6. Following exposure to purified rMG309c, vaginal and ecto- and endocervical EC secreted proinflammatory cytokines, including interleukin-6 (IL-6) and IL-8. Vaginal EC were less responsive than cervical EC. The capacity of rMG309c to bind TLR2/6 and elicit inflammation was sensitive to proteinase K digestion and independent of traditional N-terminal lipoylation. Furthermore, the immunostimulatory capacity of rMG309c was localized specifically to a 91-amino-acid subfragment of the recombinant protein, suggesting that TLR activation is likely amino acid based. Together, these data indicated that human vaginal and cervical EC are immunologically responsive to M. genitalium and to purified rMG309c via highly expressed TLR of the genital tract. These findings provide valuable insights into the mechanisms for activation of acute-phase inflammatory responses and suggest that M. genitalium colonization of reproductive tract tissues may result in inflammatory sequelae.
Figures
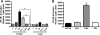
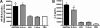
References
-
- Baggiolini, M., and I. Clark-Lewis. 1992. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 30797-101. - PubMed
-
- Baseman, J. B., S. P. Reddy, and S. F. Dallo. 1996. Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections. Am. J. Respir. Crit. Care Med. 154S137-S144. - PubMed
-
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 2561604-1607. - PubMed
-
- Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2006. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J. Biol. Chem. 2819049-9057. - PubMed
-
- Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, and A. J. Ulmer. 2005. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 2726354-6364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

