CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites
- PMID: 19103809
- PMCID: PMC2606963
- DOI: 10.1083/jcb.200809190
CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites
Abstract
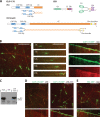
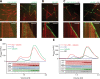
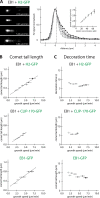
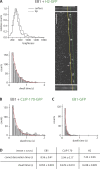
The microtubule cytoskeleton is crucial for the internal organization of eukaryotic cells. Several microtubule-associated proteins link microtubules to subcellular structures. A subclass of these proteins, the plus end-binding proteins (+TIPs), selectively binds to the growing plus ends of microtubules. Here, we reconstitute a vertebrate plus end tracking system composed of the most prominent +TIPs, end-binding protein 1 (EB1) and CLIP-170, in vitro and dissect their end-tracking mechanism. We find that EB1 autonomously recognizes specific binding sites present at growing microtubule ends. In contrast, CLIP-170 does not end-track by itself but requires EB1. CLIP-170 recognizes and turns over rapidly on composite binding sites constituted by end-accumulated EB1 and tyrosinated alpha-tubulin. In contrast to its fission yeast orthologue Tip1, dynamic end tracking of CLIP-170 does not require the activity of a molecular motor. Our results demonstrate evolutionary diversity of the plus end recognition mechanism of CLIP-170 family members, whereas the autonomous end-tracking mechanism of EB family members is conserved.
Figures
Comment in
-
EBs clip CLIPs to growing microtubule ends.J Cell Biol. 2008 Dec 29;183(7):1183-5. doi: 10.1083/jcb.200811136. Epub 2008 Dec 22. J Cell Biol. 2008. PMID: 19103811 Free PMC article.
References
-
- Akhmanova, A., and M.O. Steinmetz. 2008. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9:309–322. - PubMed
-
- Arnal, I., C. Heichette, G.S. Diamantopoulos, and D. Chretien. 2004. CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr. Biol. 14:2086–2095. - PubMed
-
- Bieling, P., L. Laan, H. Schek, E.L. Munteanu, L. Sandblad, M. Dogterom, D. Brunner, and T. Surrey. 2007. Reconstitution of a microtubule plus-end tracking system in vitro. Nature. 450:1100–1105. - PubMed
-
- Busch, K.E., J. Hayles, P. Nurse, and D. Brunner. 2004. Tea2p kinesin is involved in spatial microtubule organization by transporting tip1p on microtubules. Dev. Cell. 6:831–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

