Imaging the glycome
- PMID: 19104067
- PMCID: PMC2629201
- DOI: 10.1073/pnas.0811481106
Imaging the glycome
Abstract
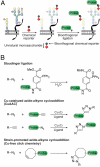
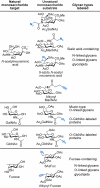
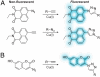
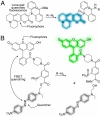
Molecular imaging enables visualization of specific molecules in vivo and without substantial perturbation to the target molecule's environment. Glycans are appealing targets for molecular imaging but are inaccessible with conventional approaches. Classic methods for monitoring glycans rely on molecular recognition with probe-bearing lectins or antibodies, but these techniques are not well suited to in vivo imaging. In an emerging strategy, glycans are imaged by metabolic labeling with chemical reporters and subsequent ligation to fluorescent probes. This technique has enabled visualization of glycans in living cells and in live organisms such as zebrafish. Molecular imaging with chemical reporters offers a new avenue for probing changes in the glycome that accompany development and disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. - PubMed
-
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. - PubMed
-
- Lecuyer E, et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–187. - PubMed
-
- Villalobos V, Naik S, Piwnica-Worms D. Current state of imaging protein-protein interactions in vivo with genetically encoded reporters. Annu Rev Biomed Eng. 2007;9:321–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

