Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages
- PMID: 19104081
- PMCID: PMC2652374
- DOI: 10.1182/blood-2008-03-146720
Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages
Abstract
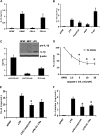
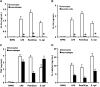
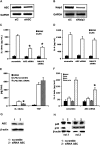
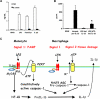
The processing of pro-interleukin-1beta depends on activation of caspase-1. Controversy has arisen whether Toll-like receptor (TLR) ligands alone can activate caspase-1 for release of interleukin-1beta (IL-1beta). Here we demonstrate that human blood monocytes release processed IL-1beta after a one-time stimulation with either TLR2 or TLR4 ligands, resulting from constitutively activated caspase-1 and release of endogenous adenosine triphosphate. The constitutive activation of caspase-1 depends on the inflammasome components, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and NALP3, but in monocytes caspase-1 activation is uncoupled from pathogen-associated molecular pattern recognition. In contrast, macrophages are unable to process and release IL-1beta solely by TLR ligands and require a second adenosine triphosphate stimulation. We conclude that IL-1beta production is differentially regulated in monocytes and macrophages, and this reflects their separate functions in host defense and inflammation.
Figures



References
-
- Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–612. - PubMed
-
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. - PMC - PubMed
-
- van der Meer JW, Vossen JM, Radl J, et al. Hyperimmunoglobulinaemia D and periodic fever: a new syndrome. Lancet. 1984;1:1087–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

