Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis
- PMID: 19104151
- PMCID: PMC2613458
- DOI: 10.1172/JCI35440
Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis
Abstract
Oligodendrocyte precursor cells (OPCs) persist near the demyelinated axons arising in MS but inefficiently differentiate into oligodendrocytes and remyelinate these axons. The pathogenesis of differentiation failure remains elusive. We initially hypothesized that injured axons fail to present Contactin, a positive ligand for the oligodendroglial Notch1 receptor to induce myelination, and thus tracked axoglial Contactin/Notch1 signaling in situ, using immunohistochemistry in brain tissue from MS patients containing chronic demyelinated lesions. Instead, we found that Contactin was saturated on demyelinated axons, Notch1-positive OPCs accumulated in Contactin-positive lesions, and the receptor was engaged, as demonstrated by cleavage to Notch1-intracellular domain (NICD). However, nuclear translocalization of NICD, required for myelinogenesis, was virtually absent in these cells. NICD and related proteins carrying nuclear localization signals were associated with the nuclear transporter Importin but were trapped in the cytoplasm. Abnormal expression of TIP30, a direct inhibitor of Importin, was observed in these OPCs. Overexpression of TIP30 in a rat OPC cell line resulted in cytoplasmic entrapment of NICD and arrest of differentiation upon stimulation with Contactin-Fc. Our results suggest that extracellular inhibitory factors as well as an intrinsic nucleocytoplasmic transport blockade within OPCs may be involved in the pathogenesis of remyelination failure in MS.
Figures
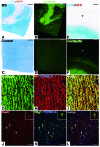
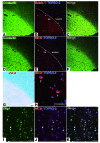
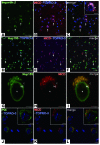


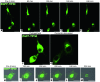


Comment in
-
Revisiting Notch in remyelination of multiple sclerosis lesions.J Clin Invest. 2009 Jan;119(1):10-3. doi: 10.1172/JCI37786. J Clin Invest. 2009. PMID: 19104146 Free PMC article.
Similar articles
-
Revisiting Notch in remyelination of multiple sclerosis lesions.J Clin Invest. 2009 Jan;119(1):10-3. doi: 10.1172/JCI37786. J Clin Invest. 2009. PMID: 19104146 Free PMC article.
-
Factors that retard remyelination in multiple sclerosis with a focus on TIP30: a novel therapeutic target.Expert Opin Ther Targets. 2009 Dec;13(12):1375-86. doi: 10.1517/14728220903307491. Expert Opin Ther Targets. 2009. PMID: 19839715 Review.
-
[Novel remyelination strategy for multiple sclerosis in the era of oligodendrocytopathy].Rinsho Shinkeigaku. 2012;52(11):1351-3. doi: 10.5692/clinicalneurol.52.1351. Rinsho Shinkeigaku. 2012. PMID: 23196615 Japanese.
-
TIP30 inhibits oligodendrocyte precursor cell differentiation via cytoplasmic sequestration of Olig1.Glia. 2015 Apr;63(4):684-98. doi: 10.1002/glia.22778. Epub 2014 Dec 19. Glia. 2015. PMID: 25530119
-
Cross-talk between F3/contactin and Notch at axoglial interface: a role in oligodendrocyte development.Dev Neurosci. 2006;28(1-2):25-33. doi: 10.1159/000090750. Dev Neurosci. 2006. PMID: 16508301 Review.
Cited by
-
Intracellular Protein Shuttling: A Mechanism Relevant for Myelin Repair in Multiple Sclerosis?Int J Mol Sci. 2015 Jul 3;16(7):15057-85. doi: 10.3390/ijms160715057. Int J Mol Sci. 2015. PMID: 26151843 Free PMC article. Review.
-
Serum contactin-1 as a biomarker of long-term disease progression in natalizumab-treated multiple sclerosis.Mult Scler. 2022 Jan;28(1):102-110. doi: 10.1177/13524585211010097. Epub 2021 Apr 23. Mult Scler. 2022. PMID: 33890520 Free PMC article.
-
New Epidermal-Growth-Factor-Related Insights Into the Pathogenesis of Multiple Sclerosis: Is It Also Epistemology?Front Neurol. 2021 Nov 26;12:754270. doi: 10.3389/fneur.2021.754270. eCollection 2021. Front Neurol. 2021. PMID: 34899572 Free PMC article. Review.
-
CC3/TIP30 regulates metabolic adaptation of tumor cells to glucose limitation.Cell Cycle. 2010 Dec 15;9(24):4941-53. doi: 10.4161/cc.9.24.14230. Epub 2010 Dec 15. Cell Cycle. 2010. PMID: 21150275 Free PMC article.
-
RNA-binding protein altered expression and mislocalization in MS.Neurol Neuroimmunol Neuroinflamm. 2020 Mar 26;7(3):e704. doi: 10.1212/NXI.0000000000000704. Print 2020 May. Neurol Neuroimmunol Neuroinflamm. 2020. PMID: 32217641 Free PMC article.

