Expression profile of nerve growth factor after muscle incision in the rat
- PMID: 19104181
- PMCID: PMC2727137
- DOI: 10.1097/ALN.0b013e318190bc84
Expression profile of nerve growth factor after muscle incision in the rat
Abstract
Background: Previous studies have demonstrated that nerve growth factor (NGF) is an important mediator of pathologic pain. Many studies have focused on cutaneous mechanisms for NGF-induced hyperalgesia; few have examined its contribution in deeper tissues like muscle. This study examined pain behaviors and the expression of NGF in incised hind paw flexor digitorum brevis muscle.
Methods: Adult Sprague-Dawley rats were pretreated with anti-NGF peptibody and underwent skin or skin plus deep fascia and muscle incision. Guarding pain behaviors were measured. Muscle NGF messenger RNA (mRNA) was measured by reverse-transcriptase polymerase chain reaction. Changes in NGF protein expression were measured using Western blot, enzyme-linked immunosorbent assay, and immunohistochemistry. In situ hybridization for NGF mRNA was also performed.
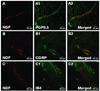
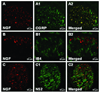
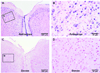
Results: Pretreatment with anti-NGF peptibody (100 mg/kg) decreased the guarding behavior caused by deep fascia and muscle incision. Muscle NGF mRNA increased abruptly 2 h after incision and was the same as control by postoperative day 1. NGF protein increased from 4 h after incision and was sustained for several days. NGF was localized in many calcitonin gene-related peptide-positive axons, few N52-positive axons, but not isolectin B4-positive axons in incised muscle. The sources of NGF mRNA included keratinocytes in epidermis and fibroblasts in deeper tissues.
Conclusion: Fibroblasts adjacent to the injury are sources of NGF in incised muscle. NGF is upregulated by incision of muscle and contributes to guarding pain behavior.
Conflict of interest statement
Figures





Similar articles
-
Nerve growth factor expression after plantar incision in the rat.Anesthesiology. 2007 Jul;107(1):128-35. doi: 10.1097/01.anes.0000267512.08619.bd. Anesthesiology. 2007. PMID: 17585224
-
Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia.Pain. 2005 Sep;117(1-2):68-76. doi: 10.1016/j.pain.2005.05.017. Pain. 2005. PMID: 16061324
-
Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain.Anesthesiology. 2004 Aug;101(2):468-75. doi: 10.1097/00000542-200408000-00029. Anesthesiology. 2004. PMID: 15277931
-
Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model.J Neurosci. 2001 Jul 1;21(13):4891-900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. J Neurosci. 2001. PMID: 11425916 Free PMC article.
-
Muscle Reactive Oxygen Species (ROS) Contribute to Post-Incisional Guarding via the TRPA1 Receptor.PLoS One. 2017 Jan 19;12(1):e0170410. doi: 10.1371/journal.pone.0170410. eCollection 2017. PLoS One. 2017. PMID: 28103292 Free PMC article.
Cited by
-
Role for NGF in augmented sympathetic nerve response to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion.J Appl Physiol (1985). 2012 Oct 15;113(8):1311-22. doi: 10.1152/japplphysiol.00617.2012. Epub 2012 Jun 28. J Appl Physiol (1985). 2012. PMID: 22744968 Free PMC article.
-
Neurobiology of fibromyalgia and chronic widespread pain.Neuroscience. 2016 Dec 3;338:114-129. doi: 10.1016/j.neuroscience.2016.06.006. Epub 2016 Jun 9. Neuroscience. 2016. PMID: 27291641 Free PMC article. Review.
-
Skin incision induces expression of axonal regeneration-related genes in adult rat spinal sensory neurons.J Pain. 2010 Nov;11(11):1066-73. doi: 10.1016/j.jpain.2010.02.001. Epub 2010 Jun 2. J Pain. 2010. PMID: 20627820 Free PMC article.
-
Mitogen-activated protein kinase phosphatase-3 (MKP-3) in the surgical wound is necessary for the resolution of postoperative pain in mice.J Pain Res. 2017 Mar 28;10:763-774. doi: 10.2147/JPR.S129826. eCollection 2017. J Pain Res. 2017. PMID: 28405172 Free PMC article.
-
Nociceptive Sensitization by Activation of Protease-Activated Receptor 2 in a Rat Model of Incisional Pain.Brain Sci. 2021 Jan 22;11(2):144. doi: 10.3390/brainsci11020144. Brain Sci. 2021. PMID: 33499207 Free PMC article.
References
-
- Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A.Update of the NGF saga J Neurol Sci 1995130119 0127 - PubMed
-
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. - PubMed
-
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. - PubMed
-
- Petty BG, Cornblath DR, Adornato BT, Chaudhry V, Flexner C, Wachsman M, Sinicropi D, Burton LE, Peroutka SJ. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol. 1994;36:244–246. - PubMed
-
- Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O'Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

