Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship
- PMID: 19104448
- PMCID: PMC2692822
- DOI: 10.1097/NEN.0b013e3181919a48
Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship
Abstract
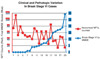
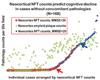
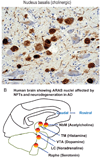
Amyloid plaques and neurofibrillary tangles (NFTs) are the pathological hallmarks of Alzheimer disease (AD). There is controversy regarding the use of current diagnostic criteria for AD and whether amyloid plaques and NFTs contribute to cognitive impairment. Because AD is specific to humans, rigorous and comprehensiveclinicopathologic studies are necessary to test and refine hypotheses of AD diagnosis and pathogenesis. Neither the clinical nor the pathological aspects of AD evolve in a linear manner, but thepredictable sequence of AD pathology allows for stage-based correlations with cognitive deterioration. We discuss subsets of patients with clinical dementia who lack amyloid plaques and NFTs and, conversely, whether individuals without antemortem cognitive impairment can harbor severe AD-type pathological findings at autopsy. There are many medical, technical, and anatomical challenges to clinicopathologic studies in AD. For example, at least two thirds of persons older than 80 years have non-AD brain diseases that can effect on cognitive function. We argue that existing data strongly support the hypothesis that both amyloid plaques and NFTs contribute to cognitive impairment.
Figures
References
-
- Goedert M. Tau protein and neurodegeneration. Semin Cell Dev Biol. 2004;15:45–49. - PubMed
-
- Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. - PubMed
-
- Nelson PT, Keller JN. RNA in brain disease: No longer just “the messenger in the middle.”. J Neuropathol Exp Neurol. 2007;66:461–468. - PubMed
-
- Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: A comparative study and review. Ann Neurol. 1996;40:139–148. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

