Development and validation of a severity scale for leprosy type 1 reactions
- PMID: 19104651
- PMCID: PMC2596969
- DOI: 10.1371/journal.pntd.0000351
Development and validation of a severity scale for leprosy type 1 reactions
Abstract
Objectives: To develop a valid and reliable quantitative measure of leprosy Type 1 reactions.
Methods: A scale was developed from previous scales which had not been validated. The face and content validity were assessed following consultation with recognised experts in the field. The construct validity was determined by applying the scale to patients in Bangladesh and Brazil who had been diagnosed with leprosy Type 1 reaction. An expert categorized each patient's reaction as mild or moderate or severe. Another worker applied the scale. This was done independently. In a subsequent stage of the study the agreement between two observers was assessed.
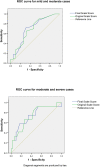
Results: The scale had good internal consistency demonstrated by a Cronbach's alpha >0.8. Removal of three items from the original scale resulted in better discrimination between disease severity categories. Cut off points for Type 1 reaction severities were determined using Receiver Operating Characteristic curves. A mild Type 1 reaction is characterized using the final scale by a score of 4 or less. A moderate reaction is a score of between 4.5 and 8.5. A severe reaction is a score of 9 or more.
Conclusions: We have developed a valid and reliable tool for quantifying leprosy Type 1 reaction severity and believe this will be a useful tool in research of this condition, in observational and intervention studies, and in the comparison of clinical and laboratory parameters.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Global leprosy situation, beginning of 2008. Wkly Epidemiol Rec. 2008;83:293–300. - PubMed
-
- Ridley DS, Jopling WH. Classification of Leprosy according to immunity. Int J Lepr Other Mycobact Dis. 1966;34:255–273. - PubMed
-
- Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1994;102:828–832. - PubMed
-
- van Brakel WH, Nicholls PG, Das L, Barkataki P, Suneetha SK, et al. The INFIR Cohort Study: investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in north India. Lepr Rev. 2005;76:14–34. - PubMed
-
- Ranque B, Nguyen VT, Vu HT, Nguyen TH, Nguyen NB, et al. Age is an important risk factor for onset and sequelae of reversal reactions in Vietnamese patients with leprosy. Clin Infect Dis. 2007;44:33–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

