A recurrent network in the lateral amygdala: a mechanism for coincidence detection
- PMID: 19104668
- PMCID: PMC2605401
- DOI: 10.3389/neuro.04.003.2008
A recurrent network in the lateral amygdala: a mechanism for coincidence detection
Abstract
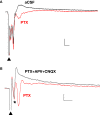
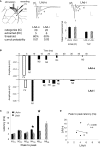
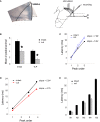
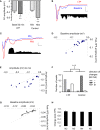
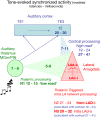
Synaptic changes at sensory inputs to the dorsal nucleus of the lateral amygdala (LAd) play a key role in the acquisition and storage of associative fear memory. However, neither the temporal nor spatial architecture of the LAd network response to sensory signals is understood. We developed a method for the elucidation of network behavior. Using this approach, temporally patterned polysynaptic recurrent network responses were found in LAd (intra-LA), both in vitro and in vivo, in response to activation of thalamic sensory afferents. Potentiation of thalamic afferents resulted in a depression of intra-LA synaptic activity, indicating a homeostatic response to changes in synaptic strength within the LAd network. Additionally, the latencies of thalamic afferent triggered recurrent network activity within the LAd overlap with known later occurring cortical afferent latencies. Thus, this recurrent network may facilitate temporal coincidence of sensory afferents within LAd during associative learning.
Keywords: Hopfield; LTD; autoassociative; feedback; polysynaptic; reverberation.
Figures



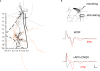


Similar articles
-
Hebbian reverberations in emotional memory micro circuits.Front Neurosci. 2009 Sep 15;3(2):198-205. doi: 10.3389/neuro.01.027.2009. eCollection 2009 Sep. Front Neurosci. 2009. PMID: 20011142 Free PMC article.
-
Fear Learning: An Evolving Picture for Plasticity at Synaptic Afferents to the Amygdala.Neuroscientist. 2024 Feb;30(1):87-104. doi: 10.1177/10738584221108083. Epub 2022 Jul 13. Neuroscientist. 2024. PMID: 35822657 Review.
-
Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons.J Physiol. 2015 Oct 1;593(19):4387-409. doi: 10.1113/JP270645. Epub 2015 Aug 30. J Physiol. 2015. PMID: 26227545 Free PMC article.
-
Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala.Eur J Neurosci. 2009 Dec 3;30(11):2089-99. doi: 10.1111/j.1460-9568.2009.07004.x. Epub 2009 Nov 25. Eur J Neurosci. 2009. PMID: 20128847
-
Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory.Neuropharmacology. 2007 Jan;52(1):215-27. doi: 10.1016/j.neuropharm.2006.06.022. Epub 2006 Aug 21. Neuropharmacology. 2007. PMID: 16919687 Review.
Cited by
-
Characterization of mWake expression in the murine brain.J Comp Neurol. 2021 Jun;529(8):1954-1987. doi: 10.1002/cne.25066. Epub 2020 Nov 10. J Comp Neurol. 2021. PMID: 33140455 Free PMC article.
-
An amygdalar oscillator coordinates cellular and behavioral rhythms.Neuron. 2024 Nov 20;112(22):3750-3767.e7. doi: 10.1016/j.neuron.2024.08.013. Epub 2024 Sep 19. Neuron. 2024. PMID: 39303704
-
Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala.Nat Neurosci. 2011 Dec 11;15(1):113-22. doi: 10.1038/nn.2993. Nat Neurosci. 2011. PMID: 22158512 Free PMC article.
-
PTEN knockdown alters dendritic spine/protrusion morphology, not density.J Comp Neurol. 2014 Apr 1;522(5):1171-90. doi: 10.1002/cne.23488. J Comp Neurol. 2014. PMID: 24264880 Free PMC article.
-
Capsaicin-induced changes in LTP in the lateral amygdala are mediated by TRPV1.PLoS One. 2011 Jan 13;6(1):e16116. doi: 10.1371/journal.pone.0016116. PLoS One. 2011. PMID: 21249195 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

