The JIP3 scaffold protein UNC-16 regulates RAB-5 dependent membrane trafficking at C. elegans synapses
- PMID: 19105215
- PMCID: PMC2707823
- DOI: 10.1002/dneu.20690
The JIP3 scaffold protein UNC-16 regulates RAB-5 dependent membrane trafficking at C. elegans synapses
Abstract
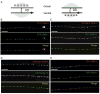
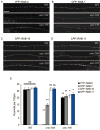
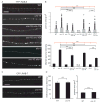
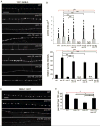
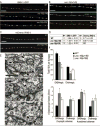
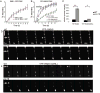
How endosomes contribute to the maintenance of vesicular structures at presynaptic terminals remains controversial and poorly understood. Here, we have investigated synaptic endosomal compartments in the presynaptic terminals of C. elegans GABAergic motor neurons. Using RAB reporters, we find that several subsynaptic compartments reside in, or near, presynaptic regions. Loss of function in the C. elegans JIP3 protein, UNC-16, causes a RAB-5-containing compartment to accumulate abnormally at presynaptic terminals. Ultrastructural analysis shows that synapses in unc-16 mutants contain reduced number of synaptic vesicles, accompanied by an increase in the size and number of cisternae. FRAP analysis revealed a slow recovery of RAB-5 in unc-16 mutants, suggestive of an impairment of RAB-5 activity state and local vesicular trafficking. Overexpression of RAB-5:GDP partially suppresses, whereas overexpression of RAB-5:GTP enhances, the synaptic defects of unc-16 mutants. Our data demonstrate a novel function of UNC-16 in the regulation of synaptic membrane trafficking and suggest that the synaptic RAB-5 compartment contributes to synaptic vesicle biogenesis or maintenance.
Figures

References
-
- Bonanomi D, Benfenati F, Valtorta F. Protein sorting in the synaptic vesicle life cycle. Prog Neurobiol. 2006;80:177–217. - PubMed
-
- Hallam SJ, Jin Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature. 1998;395:78–82. - PubMed
-
- Hamill DR, Severson AF, Carter JC, Bowerman B. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev Cell. 2002;3:673–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

