The inhibition of assembly of HIV-1 virus-like particles by 3-O-(3',3'-dimethylsuccinyl) betulinic acid (DSB) is counteracted by Vif and requires its Zinc-binding domain
- PMID: 19105849
- PMCID: PMC2628355
- DOI: 10.1186/1743-422X-5-162
The inhibition of assembly of HIV-1 virus-like particles by 3-O-(3',3'-dimethylsuccinyl) betulinic acid (DSB) is counteracted by Vif and requires its Zinc-binding domain
Abstract
Background: DSB, the 3-O-(3',3'dimethylsuccinyl) derivative of betulinic acid, blocks the last step of protease-mediated processing of HIV-1 Gag precursor (Pr55Gag), which leads to immature, noninfectious virions. When administered to Pr55Gag-expressing insect cells (Sf9), DSB inhibits the assembly and budding of membrane-enveloped virus-like particles (VLP). In order to explore the possibility that viral factors could modulate the susceptibility to DSB of the VLP assembly process, several viral proteins were coexpressed individually with Pr55Gag in DSB-treated cells, and VLP yields assayed in the extracellular medium.
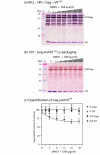
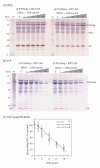
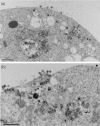
Results: Wild-type Vif (Vifwt) restored the VLP production in DSB-treated cells to levels observed in control, untreated cells. DSB-counteracting effect was also observed with Vif mutants defective in encapsidation into VLP, suggesting that packaging and anti-DSB effect were separate functions in Vif. The anti-DSB effect was abolished for VifC133S and VifS116V, two mutants which lacked the zinc binding domain (ZBD) formed by the four H(108)C(114)C(133)H(139) coordinates with a Zn atom. Electron microscopic analysis of cells coexpressing Pr55Gag and Vifwt showed that a large proportion of VLP budded into cytoplasmic vesicles and were released from Sf9 cells by exocytosis. However, in the presence of mutant VifC133S or VifS116V, most of the VLP assembled and budded at the plasma membrane, as in control cells expressing Pr55Gag alone.
Conclusion: The function of HIV-1 Vif protein which negated the DSB inhibition of VLP assembly was independent of its packaging capability, but depended on the integrity of ZBD. In the presence of Vifwt, but not with ZBD mutants VifC133S and VifS116V, VLP were redirected to a vesicular compartment and egressed via the exocytic pathway.
Figures






References
-
- Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci USA. 2003;100:13555–13560. - PMC - PubMed
-
- Li F, Zoumplis D, Matallana C, Kilgore NR, Reddick M, Yunus AS, Adamson CS, Salzwedel K, Martin DE, Allaway GP, Freed EO, Wild CT. Determinants of activity of the HIV-1 maturation inhibitor PA-457. Virology. 2006;356:217–224. - PubMed
-
- Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39:1016–1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

