Glycogen synthase kinase-3 regulates the phosphorylation of severe acute respiratory syndrome coronavirus nucleocapsid protein and viral replication
- PMID: 19106108
- PMCID: PMC8011290
- DOI: 10.1074/jbc.M805747200
Glycogen synthase kinase-3 regulates the phosphorylation of severe acute respiratory syndrome coronavirus nucleocapsid protein and viral replication
Abstract
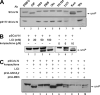
Coronavirus (CoV) nucleocapsid (N) protein is a highly phosphorylated protein required for viral replication, but whether its phosphorylation and the related kinases are involved in the viral life cycle is unknown. We found the severe acute respiratory syndrome CoV N protein to be an appropriate system to address this issue. Using high resolution PAGE analysis, this protein could be separated into phosphorylated and unphosphorylated isoforms. Mass spectrometric analysis and deletion mapping showed that the major phosphorylation sites were located at the central serine-arginine (SR)-rich motif that contains several glycogen synthase kinase (GSK)-3 substrate consensus sequences. GSK-3-specific inhibitor treatment dephosphorylated the N protein, and this could be recovered by the constitutively active GSK-3 kinase. Immunoprecipitation brought down both N and GSK-3 proteins in the same complex, and the N protein could be phosphorylated directly at its SR-rich motif by GSK-3 using an in vitro kinase assay. Mutation of the two priming sites critical for GSK-3 phosphorylation in the SR-rich motif abolished N protein phosphorylation. Finally, GSK-3 inhibitor was found to reduce N phosphorylation in the severe acute respiratory syndrome CoV-infected VeroE6 cells and decrease the viral titer and cytopathic effects. The effect of GSK-3 inhibitor was reproduced in another coronavirus, the neurotropic JHM strain of mouse hepatitis virus. Our results indicate that GSK-3 is critical for CoV N protein phosphorylation and suggest that it plays a role in regulating the viral life cycle. This study, thus, provides new avenues to further investigate the specific role of N protein phosphorylation in CoV replication.
Figures





References
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
-
- Fang X., Ye L., Timani K.A., Li S., Zen Y., Zhao M., Zheng H., Wu Z. J. Biochem. Mol. Biol. 2005;38:381–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

