Platelet-derived growth factor selectively inhibits NR2B-containing N-methyl-D-aspartate receptors in CA1 hippocampal neurons
- PMID: 19106110
- PMCID: PMC2658099
- DOI: 10.1074/jbc.M805384200
Platelet-derived growth factor selectively inhibits NR2B-containing N-methyl-D-aspartate receptors in CA1 hippocampal neurons
Abstract
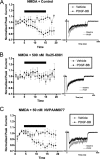
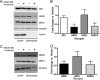
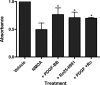
Platelet-derived growth factor (PDGF) beta receptor activation inhibits N-methyl-d-aspartate (NMDA)-evoked currents in hippocampal and cortical neurons via the activation of phospholipase Cgamma, PKC, the release of intracellular calcium, and a rearrangement of the actin cytoskeleton. In the hippocampus, the majority of NMDA receptors are heteromeric; most are composed of 2 NR1 subunits and 2 NR2A or 2 NR2B subunits. Using NR2B- and NR2A-specific antagonists, we demonstrate that PDGF-BB treatment preferentially inhibits NR2B-containing NMDA receptor currents in CA1 hippocampal neurons and enhances long-term depression in an NR2B subunit-dependent manner. Furthermore, treatment of hippocampal slices or cultures with PDGF-BB decreases the surface localization of NR2B but not of NR2A subunits. PDGFbeta receptors colocalize to a higher degree with NR2B subunits than with NR2A subunits. After neuronal injury, PDGFbeta receptors and PDGF-BB are up-regulated and PDGFbeta receptor activation is neuroprotective against glutamate-induced neuronal damage in cultured neurons. We demonstrate that the neuroprotective effects of PDGF-BB are occluded by the NR2B antagonist, Ro25-6981, and that PDGF-BB promotes NMDA signaling to CREB and ERK1/2. We conclude that PDGFbetaR signaling, by preferentially targeting NR2B receptors, provides an important mechanism for neuroprotection by growth factors in the central nervous system.
Figures


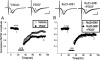

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

