Subdiaphragmatic vagotomy prevents drinking-induced reduction in plasma corticosterone in water-restricted rats
- PMID: 19106215
- PMCID: PMC2671899
- DOI: 10.1210/en.2008-1594
Subdiaphragmatic vagotomy prevents drinking-induced reduction in plasma corticosterone in water-restricted rats
Abstract
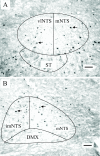
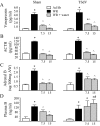
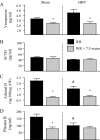
Dehydrated rats exhibit a rapid inhibition of the hypothalamic-pituitary-adrenal axis after rehydration. Drinking activates vagal afferents that project to neurons in the nucleus tractus solitarius (NTS). We hypothesized that when dehydrated rats drink, vagal afferents stimulate NTS neurons initiating inhibition of hypothalamic-pituitary-adrenal activity. Experiments assessed NTS activity by measuring Fos expression. Rats were water restricted for 1 or 6 d, limiting access to water to 30 min/d in the morning. Drinking after single or repeated restriction increased Fos, demonstrating increased NTS activity. We next examined the contribution of the vagus by comparing hormonal responses after total subdiaphragmatic vagotomy or sham surgery. Water restriction for 6 d increased plasma arginine vasopressin (AVP), ACTH, and adrenal and plasma corticosterone in both groups. In sham rats, drinking reduced plasma AVP, ACTH, adrenal and plasma corticosterone by 7.5 min. In total subdiaphragmatic vagotomy rats, whereas drinking reduced plasma AVP, ACTH, and adrenal corticosterone, drinking did not reduce plasma corticosterone. To identify the source of vagal activity, hormonal responses to restriction-induced drinking were measured after common hepatic branch vagotomy (HBV). Although pituitary hormonal responses were not affected by HBV, the adrenal and plasma corticosterone responses to water restriction were reduced; in addition, drinking in HBV rats decreased adrenal corticosterone without changing plasma corticosterone. These data indicate that an intact vagus is necessary to reduce plasma corticosterone when water-restricted rats drink and that the common hepatic vagal branch contributes to the response. These findings implicate the vagus in augmenting rapid removal of circulating corticosterone during relief from stress.
Figures

References
-
- Tsigosa C, Chrousos GP 2002 Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53:865–871 - PubMed
-
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE 2007 The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn 65:209–237 - PubMed
-
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE 2003 Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 - PubMed
-
- Chrousos GP, Gold PW 1998 A healthy body in a healthy mind—and vice versa—the damaging power of “uncontrollable” stress. J Clin Endocrinol Metab 83:1842–1845 - PubMed
-
- Gray GD, Bergfors R, Levin R, Levine S 1978 Comparisons of the effect of restricted morning or evening water intake on adrenocortical activity in female rats. Neuroendocrinology 25:236–246 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

