Mice lacking asparaginyl endopeptidase develop disorders resembling hemophagocytic syndrome
- PMID: 19106291
- PMCID: PMC2626726
- DOI: 10.1073/pnas.0809824105
Mice lacking asparaginyl endopeptidase develop disorders resembling hemophagocytic syndrome
Erratum in
- Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):6023. Zheng, Chengyun [added]; Henter, Jan-Inge [added]; Meeths, Marie [added]; Nordenskjold, Magnus [added]
Abstract
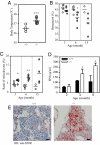
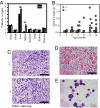
Asparaginyl endopeptidase (AEP or legumain) is a lysosomal cysteine protease that cleaves protein substrates on the C-terminal side of asparagine. AEP plays a pivotal role in the endosome/lysosomal degradation system and is implicated in antigen processing. The processing of the lysosomal proteases cathepsins in kidney is completely defective in AEP-deficient mice with accumulation of macromolecules in the lysosomes, which is typically seen in lysosomal disorders. Here we show that mutant mice lacking AEP develop fever, cytopenia, hepatosplenomegaly, and hemophagocytosis, which are primary pathological manifestations of hemophagocytic syndrome/hemophagocytic lymphohistiocytosis (HLH). Moreover, AEP deficiency provokes extramedullary hematopoiesis in the spleen and abnormally enlarged histiocytes with ingested red blood cells (RBCs) in bone marrow. Interestingly, RBCs from AEP-null mice are defective in plasma membrane components. Further, AEP-null mice display lower natural killer cell activity, but none of the major cytokines is substantially abnormal. These results indicate that AEP might be a previously unrecognized component in HLH pathophysiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166:95–109. - PubMed
-
- Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–253. - PubMed
-
- Stepp SE, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. - PubMed
-
- Feldmann J, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. - PubMed
-
- Menasche G, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

