A tobacco calcium-dependent protein kinase, CDPK1, regulates the transcription factor REPRESSION OF SHOOT GROWTH in response to gibberellins
- PMID: 19106376
- PMCID: PMC2630431
- DOI: 10.1105/tpc.107.057489
A tobacco calcium-dependent protein kinase, CDPK1, regulates the transcription factor REPRESSION OF SHOOT GROWTH in response to gibberellins
Abstract
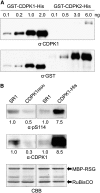
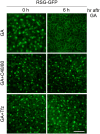
The homeostasis of gibberellins (GAs) is maintained by negative feedback in plants. REPRESSION OF SHOOT GROWTH (RSG) is a tobacco (Nicotiana tabacum) transcriptional activator that has been suggested to play a role in GA feedback by the regulation of GA biosynthetic enzymes. The 14-3-3 signaling proteins negatively regulate RSG by sequestering it in the cytoplasm in response to GAs. The phosphorylation on Ser-114 of RSG is essential for 14-3-3 binding of RSG. Here, we identified tobacco Ca(2+)-dependent protein kinase (CDPK1) as an RSG kinase that promotes 14-3-3 binding to RSG by phosphorylation of Ser-114 of RSG. CDPK1 interacts with RSG in a Ca(2+)-dependent manner in vivo and in vitro and specifically phosphorylates Ser-114 of RSG. Inhibition of CDPK repressed the GA-induced phosphorylation of Ser-114 of RSG and the GA-induced nuclear export of RSG. Overexpression of CDPK1 inhibited the feedback regulation of a GA 20-oxidase gene and resulted in sensitization to the GA biosynthetic inhibitor. Our results suggest that CDPK1 decodes the Ca(2+) signal produced by GAs and regulates the intracellular localization of RSG.
Figures
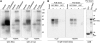
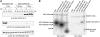



References
-
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffmann, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057. - PubMed
-
- Amador, V., Monte, E., García-Martínez, J.-L., and Prat, S. (2001). Gibberellins signal nuclear import of PHOR1, a photoperiod-responsive protein with homology to Drosophila armadillo. Cell 106 343–354. - PubMed
-
- Aoyama, T., and Chua, N.-H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11 605–612. - PubMed
-
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408 796–815. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

