Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer
- PMID: 19107118
- PMCID: PMC2835081
- DOI: 10.1038/mt.2008.281
Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer
Abstract
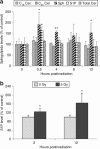
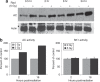
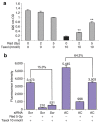
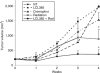
Radiation resistance in a subset of prostate tumors remains a challenge to prostate cancer radiotherapy. The current study on the effects of radiation on prostate cancer cells reveals that radiation programs an unpredicted resistance mechanism by upregulating acid ceramidase (AC). Irradiated cells demonstrated limited changes of ceramide levels while elevating levels of sphingosine and sphingosine-1-phosphate. By genetically downregulating AC with small interfering RNA (siRNA), we observed radiosensitization of cells using clonogenic and cytotoxicity assays. Conversely, AC overexpression further decreased sensitivity to radiation. We also observed that radiation-induced AC upregulation was sufficient to create cross-resistance to chemotherapy as demonstrated by decreased sensitivity to Taxol and C(6) ceramide compared to controls. Lower levels of caspase 3/7 activity were detected in cells pretreated with radiation, also indicating increased resistance. Finally, utilization of the small molecule AC inhibitor, LCL385, sensitized PPC-1 cells to radiation and significantly decreased tumor xenograft growth. These data suggest a new mechanism of cancer cell resistance to radiation, through upregulation of AC that is, in part, mediated by application of the therapy itself. An improved understanding of radiotherapy and the application of combination therapy achieved in this study offer new opportunities for the modulation of radiation effects in the treatment of cancer.
Figures




References
-
- Mangar SA, Huddart RA, Parker CC, Dearnaley DP, Khoo VS., and , Horwich A. Technological advances in radiotherapy for the treatment of localised prostate cancer. Eur J Cancer. 2005;41:908–921. - PubMed
-
- Peschel RE., and , Colberg JW. Surgery, brachytherapy, and external-beam radiotherapy for early prostate cancer. Lancet Oncol. 2003;4:233–241. - PubMed
-
- Hanks GE, Hanlon AL, Epstein B., and , Horwitz EM. Dose response in prostate cancer with 8–12 years' follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. - PubMed
-
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. - PubMed
-
- Allan DJ. Radiation-induced apoptosis: its role in a MADCaT (mitosis-apoptosis-differentiation-calcium toxicity) scheme of cytotoxicity mechanisms. Int J Radiat Biol. 1992;62:145–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

