HSP60 as a target of anti-ergotypic regulatory T cells
- PMID: 19107191
- PMCID: PMC2602852
- DOI: 10.1371/journal.pone.0004026
HSP60 as a target of anti-ergotypic regulatory T cells
Abstract
The 60 kDa heat shock protein (HSP60) has been reported to influence T-cell responses in two ways: as a ligand of toll-like receptor 2 signalling and as an antigen. Here we describe a new mechanism of T-cell immuno-regulation focused on HSP60: HSP60 is up-regulated and presented by activated T cells (HSP60 is an ergotope) to regulatory (anti-ergotypic) T cells. Presentation of HSP60 by activated T cells was found to be MHC-restricted and dependent on accessory molecules - CD28, CD80 and CD86. Anti-ergotypic T cells responded to T-cell HSP60 by proliferation and secreted IFNgamma and TGFbeta1. In vitro, the anti-ergotypic T cells inhibited IFNgamma production by their activated T-cell targets. In vivo, adoptive transfer of an anti-ergotypic HSP60-specific T-cell line led to decreased secretion of IFNgamma by arthritogenic T cells and ameliorated adjuvant arthritis (AA). Thus, the presentation of HSP60 by activated T cells turns them into targets for anti-ergotypic regulatory T cells specific for HSP60. However, the direct interaction between the anti-ergotypic T regulators (anti-HSP60) and the activated T cells also down-regulated the regulators. Thus, by functioning as an ergotope, HSP60 can control both the effector T cells and the regulatory HSP60-specific T cells that control them.
Conflict of interest statement
Figures
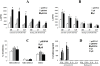


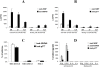
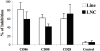


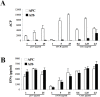
References
-
- Quintana FJ, Carmi P, Cohen IR. DNA vaccination with heat shock protein 60 inhibits cyclophosphamide-accelerated diabetes. J Immunol. 2002;169:6030–6035. - PubMed
-
- Elias D, Cohen IR. The hsp60 peptide p277 arrests the autoimmune diabetes induced by the toxin streptozotocin. Diabetes. 1996;45:1168–1172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

