Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes
- PMID: 19107653
- PMCID: PMC3612426
- DOI: 10.1080/08977190802625179
Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes
Abstract
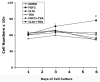
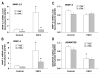
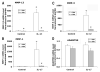
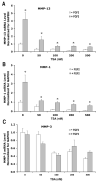
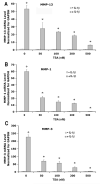
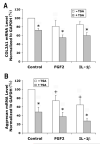
Fibroblast growth factor-2 (FGF2) and interleukin-1beta (IL-1beta) stimulate the expression of matrix metalloproteinases (MMPs) in articular chondrocytes, which may contribute to cartilage degradation and development of osteoarthritis. Histone deacetylases (HDACs) have recently been implicated in the regulation of MMP gene expression. To investigate the functional involvement of HDACs in the signaling pathway of FGF2 and IL-1beta, we examined the effects of HDAC inhibition on activities of FGF2 or IL-1beta on gene expression of MMP-1, MMP-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS5), collagen type II, and aggrecan. Human articular chondrocyte cultures were treated with FGF2 or IL-1beta in the presence or absence of HDAC inhibitor (trichostatin A, TSA). Gene expression levels after treatments were assessed using quantitative real time PCR. Results showed that FGF2 and IL-1beta both increased MMP-1 and -13 expression, while IL-1beta also increased MMP-3 mRNA levels. These effects were attenuated in the presence of TSA in a dose dependent manner. In contrast to the effects on MMPs, FGF2 decreased mRNA levels of ADAMTS-5, which was not affected by HDAC inhibition. FGF2, IL-1beta, and TSA inhibited expression of aggrecan, while TSA also decreased mRNA levels of collagen type II. These findings showed that HDAC inhibition antagonized FGF2 and IL-1beta induced MMP expression. Combination of FGF2 and the HDAC inhibitor decreases both anabolic and catabolic genes, which may slow the cartilage turnover and be beneficial for maintaining cartilage integrity.
Figures


References
-
- Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–2789. - PubMed
-
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. - PubMed
-
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
