Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria
- PMID: 19108681
- PMCID: PMC2819707
- DOI: 10.1089/ten.tec.2008.0390
Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria
Abstract
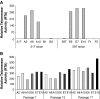
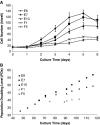
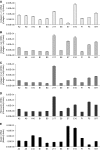
Tissue regeneration of the vocal fold lamina propria extracellular matrix (ECM) will be facilitated by the use of suitable vocal fold fibroblast (VFF) cell lines in appropriate model systems. Primary human VFFs (hVFFs) were steadily transduced by a retroviral vector containing human telomerase reverse transcriptase (hTERT) gene; immortalized cells grew and divided vigorously for more than 120 days. Biochemical characterization of the six transduced lines included, at different time points, expression of hTERT, telomerase activity, telomere lengths, and transcript levels of ECM constituents. Telomere lengths of the transfected lines were elongated and stable. Gene expression levels of collagen Ialpha1, collagen Ialpha2, collagen VIalpha3, elastin, and fibronectin were measured between the transduced cell clones and the primary hVFFs to verify transcription. Absence of inter- and intraspecies contamination was confirmed with DNA fingerprinting and karyotype analysis. Cell morphology, growth, and transcription expression were examined on 2D scaffolds-collagen, fibronectin, and hyaluronic acid. Immortalized hVFFs demonstrated normal attachment and spread on 2D scaffolds. Collagen Ialpha1, collagen Ialpha2, collagen VIalpha3, elastin, and fibronectin transcript expression was measured from immortalized hVFFs, for all surfaces. This is the first report of immortalization and biochemical characterization of hVFFs, providing a novel and invaluable tool for tissue regeneration applications in the larynx.
Figures







References
-
- Verdolini K. Ramig L. Review: occupational risks for voice problems. Logoped Phoniatr Vocol. 2001;26:37. - PubMed
-
- Ma E.P. Yiu E.M. Voice activity and participation profile: assessing the impact of voice disorders on daily activities. J Speech Lang Hear Res. 2001;44:511. - PubMed
-
- Benninger M.S. Alessi D. Archer S. Bastian R. Ford C. Koufman J. Sataloff R.T. Spiegel J.R. Woo P. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474. - PubMed
-
- Hirano M. Jackson C. Lecture. Phonosurgery: past, present, and future. American Broncho-Esophagological. 1995:Association.
-
- Johnson P.C. Mikos A.G. Fisher J.P. Jansen J.A. Strategic directions in tissue engineering. Tissue Eng. 2007;13:2827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

