CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signaling events during T lymphocyte activation
- PMID: 19109148
- PMCID: PMC2937277
- DOI: 10.4049/jimmunol.182.1.171
CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signaling events during T lymphocyte activation
Abstract
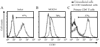
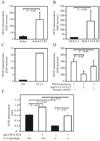
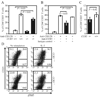
Ligands of CCR5, the major coreceptor of HIV-1, costimulate T lymphocyte activation. However, the full impact of CCR5 expression on T cell responses remains unknown. Here, we show that compared with CCR5(+/+), T cells from CCR5(-/-) mice secrete lower amounts of IL-2, and a similar phenotype is observed in humans who lack CCR5 expression (CCR5-Delta32/Delta32 homozygotes) as well as after Ab-mediated blockade of CCR5 in human T cells genetically intact for CCR5 expression. Conversely, overexpression of CCR5 in human T cells results in enhanced IL-2 production. CCR5 surface levels correlate positively with IL-2 protein and mRNA abundance, suggesting that CCR5 affects IL-2 gene regulation. Signaling via CCR5 resulted in NFAT transactivation in T cells that was blocked by Abs against CCR5 agonists, suggesting a link between CCR5 and downstream pathways that influence IL-2 expression. Furthermore, murine T cells lacking CCR5 had reduced levels of intranuclear NFAT following activation. Accordingly, CCR5 expression also promoted IL-2-dependent events, including CD25 expression, STAT5 phosphorylation, and T cell proliferation. We therefore suggest that by influencing a NFAT-mediated pathway that regulates IL-2 production and IL-2-dependent events, CCR5 may play a critical role in T cell responses. In accord with our prior inferences from genetic-epidemiologic studies, such CCR5-dependent responses might constitute a viral entry-independent mechanism by which CCR5 may influence HIV-AIDS pathogenesis.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
-
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu. Rev. Immunol. 2001;19:375–396. - PubMed
-
- Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat. Immunol. 2006;7:1101–1108. - PubMed
-
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. - PubMed
-
- Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. - PubMed
-
- Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001;2:123–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

