Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma
- PMID: 19109154
- PMCID: PMC3150499
- DOI: 10.4049/jimmunol.182.1.234
Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma
Abstract
Follistatin-like protein-1 (FSTL-1) is a poorly characterized protein that is up-regulated in the early stage of collagen-induced arthritis and that exacerbates arthritis when delivered by gene transfer. The current study was designed to determine the mechanism by which FSTL-1 promotes arthritis. FSTL-1 was injected into mouse paws, resulting in severe paw swelling associated with up-regulation of IFN-gamma transcript and the IFN-gamma-induced chemokine, CXCL10. Mice depleted of T cells were protected. A central role for IFN-gamma was confirmed by the finding that mice deficient in IFN-gamma failed to exhibit paw swelling in response to injection of FSTL-1. Furthermore, IFN-gamma secretion from mouse spleen cells exposed to a weak TCR signal was increased 5-fold in the presence of FSTL-1. FSTL-1 could be induced by innate immune signals, including TLR4 agonists and the arthritogenic cytokine, IL-1beta, via an NFkappaB pathway. Finally, FSTL-1 was found to be overexpressed in human arthritis and its neutralization inhibited murine collagen-induced arthritis and suppressed IFN-gamma and CXCL10 production in arthritic joints. These findings demonstrate that FSTL-1 plays a critical role in arthritis by enhancing IFN-gamma signaling pathways and suggest a mechanism by which FSTL-1 bridges innate and adaptive immune responses.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
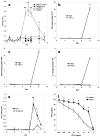
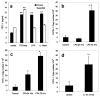
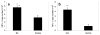
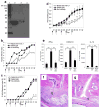
Similar articles
-
Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis.Arthritis Rheum. 2010 Aug;62(8):2510-6. doi: 10.1002/art.27485. Arthritis Rheum. 2010. PMID: 20506332 Free PMC article.
-
Follistatin-like protein-1 is a novel proinflammatory molecule.J Immunol. 2006 Oct 1;177(7):4758-62. doi: 10.4049/jimmunol.177.7.4758. J Immunol. 2006. PMID: 16982916
-
Trichostatin differentially regulates Th1 and Th2 responses and alleviates rheumatoid arthritis in mice.J Clin Immunol. 2011 Jun;31(3):395-405. doi: 10.1007/s10875-011-9508-8. Epub 2011 Feb 9. J Clin Immunol. 2011. PMID: 21305388
-
Endogenous IL-22 plays a dual role in arthritis: regulation of established arthritis via IFN-γ responses.PLoS One. 2014 Mar 27;9(3):e93279. doi: 10.1371/journal.pone.0093279. eCollection 2014. PLoS One. 2014. PMID: 24676270 Free PMC article.
-
Collagen-induced arthritis as an animal model for rheumatoid arthritis: focus on interferon-γ.J Interferon Cytokine Res. 2011 Dec;31(12):917-26. doi: 10.1089/jir.2011.0056. Epub 2011 Sep 9. J Interferon Cytokine Res. 2011. PMID: 21905879 Review.
Cited by
-
Regulation of Pulmonary Bacterial Immunity by Follistatin-Like Protein 1.Infect Immun. 2020 Dec 15;89(1):e00298-20. doi: 10.1128/IAI.00298-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33077624 Free PMC article.
-
Validation of the Diagnostic and Prognostic Values of ADAMTS5 and FSTL1 in Osteoarthritis Rat Model.Cartilage. 2021 Dec;13(2_suppl):1263S-1273S. doi: 10.1177/1947603519852405. Epub 2019 Jun 10. Cartilage. 2021. PMID: 31177809 Free PMC article.
-
Gene expression profiling in dermatitis herpetiformis skin lesions.Clin Dev Immunol. 2012;2012:198956. doi: 10.1155/2012/198956. Epub 2012 Sep 6. Clin Dev Immunol. 2012. PMID: 22991566 Free PMC article.
-
MicroRNA-27a Inhibits Cell Migration and Invasion of Fibroblast-Like Synoviocytes by Targeting Follistatin-Like Protein 1 in Rheumatoid Arthritis.Mol Cells. 2016 Aug 31;39(8):611-8. doi: 10.14348/molcells.2016.0103. Epub 2016 Aug 8. Mol Cells. 2016. PMID: 27498552 Free PMC article.
-
Data-independent acquisition of the proteomics of spleens from chickens infected by avian leukosis virus.3 Biotech. 2019 Sep;9(9):332. doi: 10.1007/s13205-019-1863-9. Epub 2019 Aug 16. 3 Biotech. 2019. PMID: 31475084 Free PMC article.
References
-
- Thornton S, Sowders D, Aronow B, Witte DP, Brunner HI, Giannini EH, Hirsch R. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin Immunol. 2002;105:155–168. - PubMed
-
- Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-β1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. - PubMed
-
- Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10:1305–1314. - PubMed
-
- Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660 – 668. - PubMed
-
- Tanaka M, Ozaki S, Kawabata D, Kishimura M, Osakada F, Okubo M, Murakami M, Nakao K, Mimori T. Potential preventive effects of follistatin-related protein/TSC-36 on joint destruction and antagonistic modulation of its autoantibodies in rheumatoid arthritis. Int Immunol. 2003;15:71–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

