Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation
- PMID: 19109157
- PMCID: PMC3731994
- DOI: 10.4049/jimmunol.182.1.259
Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation
Abstract
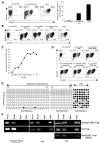
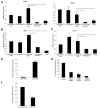
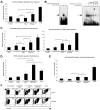
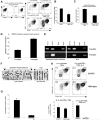
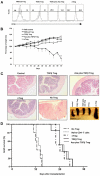
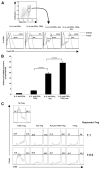
Foxp3, a winged-helix family transcription factor, serves as the master switch for CD4(+) regulatory T cells (Treg). We identified a unique and evolutionarily conserved CpG-rich island of the Foxp3 nonintronic upstream enhancer and discovered that a specific site within it was unmethylated in natural Treg (nTreg) but heavily methylated in naive CD4(+) T cells, activated CD4(+) T cells, and peripheral TGFbeta-induced Treg in which it was bound by DNMT1, DNMT3b, MeCP2, and MBD2. Demethylation of this CpG site using the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine (Aza) induced acetylation of histone 3, interaction with TIEG1 and Sp1, and resulted in strong and stable induction of Foxp3. Conversely, IL-6 resulted in methylation of this site and repression of Foxp3 expression. Aza plus TGFbeta-induced Treg resembled nTreg, expressing similar receptors, cytokines, and stable suppressive activity. Strong Foxp3 expression and suppressor activity could be induced in a variety of T cells, including human CD4(+)CD25(-) T cells. Epigenetic regulation of Foxp3 can be predictably controlled with DNMT inhibitors to generate functional, stable, and specific Treg.
Figures


References
-
- Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. - PubMed
-
- van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J. Immunol. 2002;169:5401–5404. - PubMed
-
- Wang HY, Wang RF. Regulatory T cells and cancer. Curr. Opin. Immunol. 2007;19:217–223. - PubMed
-
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

