Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells
- PMID: 19109164
- PMCID: PMC2855895
- DOI: 10.4049/jimmunol.182.1.329
Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells
Abstract
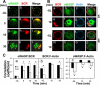
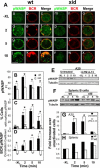
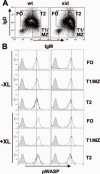
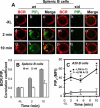
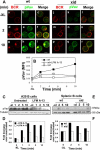
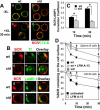
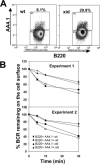
The high efficiency of Ag processing and presentation by B cells requires Ag-induced BCR signaling and actin cytoskeleton reorganization, although the underlying mechanism for such requirements remains elusive. In this study, we identify Bruton's tyrosine kinase (Btk) as a linker connecting BCR signaling to actin dynamics and the Ag transport pathway. Using xid mice and a Btk inhibitor, we show that BCR engagement increases actin polymerization and Wiskott-Aldrich syndrome protein activation in a Btk-dependent manner. Concurrently, we observe Btk-dependent increases in the levels of phosphatidylinositide-4,5-bisphosphate and phosphorylated Vav upon BCR engagement. The rate of BCR internalization, its movement to late endosomes, and efficiency of BCR-mediated Ag processing and presentation are significantly reduced in both xid and Btk inhibitor-treated B cells. Thus, Btk regulates actin dynamics and Ag transport by activating Wiskott-Aldrich syndrome protein via Vav and phosphatidylinositides. This represents a novel mechanism by which BCR-mediated signaling regulates BCR-mediated Ag processing and presentation.
Figures


References
-
- Cheng PC, Cherukuri A, Dykstra M, Malapati S, Sproul T, Chen MR, Pierce SK. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin. Immunol. 2001;13:107–114. - PubMed
-
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 1997;15:453–479. - PubMed
-
- Song W, Cho H, Cheng P, Pierce SK. Entry of B cell antigen receptor and antigen into class II peptide-loading compartment is independent of receptor cross-linking. J. Immunol. 1995;155:4255–4263. - PubMed
-
- Cheng PC, Steele CR, Gu L, Song W, Pierce SK. MHC class II antigen processing in B cells: accelerated intracellular targeting of antigens. J. Immunol. 1999;162:7171–7180. - PubMed
-
- Siemasko K, Skaggs, J. B, Kabak S, Williamson E, Brown BK, Song W, Clark, R. M. Receptor facilitated antigen presentation requires the recruitment of B cell linker protein to Igα. J. Immunol. 2002;168:2127–2138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

