Genomic instability resulting from Blm deficiency compromises development, maintenance, and function of the B cell lineage
- PMID: 19109166
- PMCID: PMC2742995
- DOI: 10.4049/jimmunol.182.1.347
Genomic instability resulting from Blm deficiency compromises development, maintenance, and function of the B cell lineage
Abstract
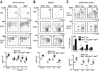
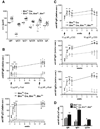
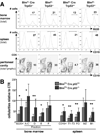
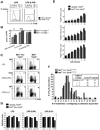
The RecQ family helicase BLM is critically involved in the maintenance of genomic stability, and BLM mutation causes the heritable disorder Bloom's syndrome. Affected individuals suffer from a predisposition to a multitude of cancer types and an ill-defined immunodeficiency involving low serum Ab titers. To investigate its role in B cell biology, we inactivated murine Blm specifically in B lymphocytes in vivo. Numbers of developing B lymphoid cells in the bone marrow and mature B cells in the periphery were drastically reduced upon Blm inactivation. Of the major peripheral B cell subsets, B1a cells were most prominently affected. In the sera of Blm-deficient naive mice, concentrations of all Ig isotypes were low, particularly IgG3. Specific IgG Ab responses upon immunization were poor and mutant B cells exhibited a generally reduced Ab class switch capacity in vitro. We did not find evidence for a crucial role of Blm in the mechanism of class switch recombination. However, a modest shift toward microhomology-mediated switch junction formation was observed in Blm-deficient B cells. Finally, a cohort of p53-deficient, conditional Blm knockout mice revealed an increased propensity for B cell lymphoma development. Impaired cell cycle progression and survival as well as high rates of chromosomal structural abnormalities in mutant B cell blasts were identified as the basis for the observed effects. Collectively, our data highlight the importance of BLM-dependent genome surveillance for B cell immunity by ensuring proper development and function of the various B cell subsets while counteracting lymphomagenesis.
Figures



References
-
- van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196–206. - PubMed
-
- Mills KD, Ferguson DO, Alt FW. The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev. 2003;194:77–95. - PubMed
-
- Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

