Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation
- PMID: 19109180
- PMCID: PMC2651083
- DOI: 10.4049/jimmunol.182.1.489
Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation
Abstract
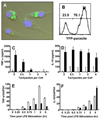
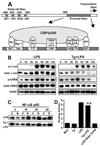
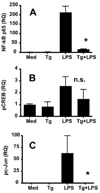
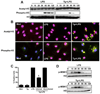
Macrophages infected with the opportunistic protozoan Toxoplasma gondii are unable to up-regulate many proinflammatory cytokine genes, including TNF (TNF-alpha), upon stimulation with LPS and other TLR ligands. In this study, we examined the influence of T. gondii on transcription factors associated with TNF-alpha transcription, as well as phosphorylation and acetylation of histone H3 at distal and proximal regions of the TNF-alpha promoter. During LPS stimulation, we found that Toxoplasma blocks nuclear accumulation of transcription factor c-Jun, but not that of cAMP response element-binding protein or NF-kappaB. However, chromatin immunoprecipitation studies revealed that binding of all of these transcription factors to the TNF promoter was decreased by T. gondii infection. Furthermore, the parasite blocked LPS-induced Ser(10) phosphorylation and Lys(9)/Lys(14) acetylation of histone H3 molecules associated with distal and proximal regions of the TNF-alpha promoter. Our results show that Toxoplasma inhibits TNF-alpha transcription by interfering with chromatin remodeling events required for transcriptional activation at the TNF promoter, revealing a new mechanism by which a eukaryotic pathogen incapacitates proinflammatory cytokine production during infection.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures



References
-
- Alexander J, Hunter CA. Immunoregulation during toxoplasmosis. Chem. Immunol. 1998;70:81–102. - PubMed
-
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 1996;157:798–805. - PubMed
-
- Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-κB. J. Immunol. 2001;167:2193–2201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

