CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation
- PMID: 19109196
- PMCID: PMC2718444
- DOI: 10.4049/jimmunol.182.1.623
CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation
Abstract
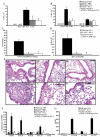
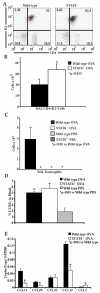
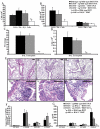
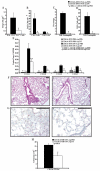
STAT6-mediated chemokine production in the lung is required for Th2 lymphocyte and eosinophil homing into the airways in allergic pulmonary inflammation, and thus is a potential therapeutic target in asthma. However, the critical cellular source of STAT6-mediated chemokine production has not been defined. In this study, we demonstrate that STAT6 in bone marrow-derived myeloid cells was sufficient for the production of CCL17, CCL22, CCL11, and CCL24 and for Th2 lymphocyte and eosinophil recruitment into the allergic airway. In contrast, STAT6 in airway-lining cells did not mediate chemokine production or support cellular recruitment. Selective depletion of CD11b(+) myeloid cells in the lung identified these cells as the critical cellular source for the chemokines CCL17 and CCL22. These data reveal that CD11b(+) myeloid cells in the lung help orchestrate the adaptive immune response in asthma, in part, through the production of STAT6-inducible chemokines and the recruitment of Th2 lymphocytes into the airway.
Figures



References
-
- Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001;344:350–362. - PubMed
-
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. quiz 464. - PubMed
-
- Woodfolk JA. T-cell responses to allergens. J Allergy Clin Immunol. 2007;119:280–294. quiz 295-286. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Del Prete GF, De Carli M, D’Elios MM, Maestrelli P, Ricci M, Fabbri L, Romagnani S. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993;23:1445–1449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

