Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages
- PMID: 19109239
- PMCID: PMC2739727
- DOI: 10.1126/scisignal.1164795
Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages
Abstract
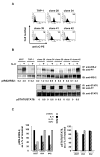
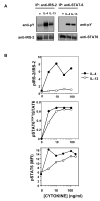
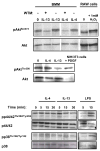
Although interleukin-4 (IL-4) and IL-13 participate in allergic inflammation and share a receptor subunit (IL-4Ralpha), they have different functions. We compared cells expressing type I and II IL-4Rs with cells expressing only type II receptors for their responsiveness to these cytokines. IL-4 induced highly efficient, gammaC-dependent tyrosine phosphorylation of insulin receptor substrate 2 (IRS-2), whereas IL-13 was less effective, even when phosphorylation of signal transducer and activator of transcription 6 (STAT6) was maximal. Only type I receptor, gammaC-dependent signaling induced efficient association of IRS-2 with the p85 subunit of phosphoinositide 3-kinase or the adaptor protein growth factor receptor-bound protein 2. In addition, IL-4 signaling through type I IL-4Rs induced more robust expression of a subset of genes associated with alternatively activated macrophages than did IL-13. Thus, IL-4 activates signaling pathways through type I IL-4Rs qualitatively differently from IL-13, which cooperate to induce optimal gene expression.
Figures






References
-
- Boulay JL, Paul WE. The interleukin-4 family of lymphokines. Curr Opin Immunol. 1992;4:294–298. - PubMed
-
- Sprang SR, Bazan JF. Cytokine structural taxonomy and mechanisms of receptor engagement. Curr Opin Struct Biol. 1993;3:815–827.
-
- Rozwarski DA, Gronenborn AM, Clore GM, Bazan JF, Bohm A, Wlodawer A, Hatada M, Karplus PA. Structural comparisons among the short-chain helical cytokines. Structure. 1994;2:159–173. - PubMed
-
- Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. - PubMed
-
- Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17:1–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

