Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8
- PMID: 19109369
- PMCID: PMC3973398
- DOI: 10.1152/ajpregu.90544.2008
Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8
Abstract
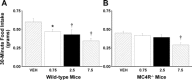
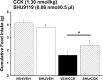
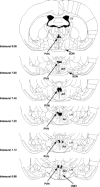
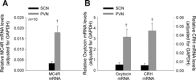
Melanocortin 4 receptors (MC4R) are hypothesized to mediate the central nervous system actions of leptin to enhance the satiety effects of cholecystokinin (CCK). To further elucidate this mechanism, we confirmed that peripheral administration of CCK-8 is less effective in producing this effect in MC4R-deficient mice (MC4R(-/-)). Whereas intraperitoneal (ip) CCK-8 at 0.75 nmol/kg lean body mass (lbm) suppressed food intake in wild-type mice, CCK-8 doses of 7.5 nmol/kg lbm were required to attenuate food intake in MC4R(-/-) mice. To determine whether melanocortin signaling in the hypothalamic paraventricular nucleus (PVN) participates in regulating this CCK satiety response, we administered the MC3/MC4R antagonist, SHU9119, into the PVN of rats before ip CCK-8 administration. PVN administration of SHU9119 attenuated the ability of CCK-8 to reduce 30-min food intake by 20%. To determine whether MC4R are expressed by PVN neurons that project directly to hindbrain nuclei involved in the satiety response to ip CCK-8, the retrograde tracer fluorescent cholera toxin subunit B was injected into the nucleus tractus solitarius (NTS) of the hindbrain. After 4 days, labeled PVN neurons were collected by laser capture microdissection and found to express MC4R mRNA by quantitative RT-PCR analysis. These data provide evidence for a neuroanatomical link between hypothalamic melanocortin signaling in the PVN and NTS neurons that regulate food intake. These findings highlight the contribution of melanocortin signaling in the PVN toward regulating the satiety effects of CCK-8 while acknowledging that melanocortin-dependent pathways in other brain regions and/or melanocortin-independent mechanisms are also important in this mechanism.
Figures


References
-
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996 - PubMed
-
- Aja SM, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol 281: R1862–R1867, 2001 - PubMed
-
- Azzara AV, Schuss B, Hong S, Chua SC, Schwartz GJ. Melanocortin 4 receptor (MC4R) knockout mice have increased food intake and meal size, and decreased sensitivity to post-oral nutrient stimulation. Appetite 44: 332, 2005
-
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav 77: 411–416, 2002 - PubMed
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

