Gag p27-specific B- and T-cell responses in Simian immunodeficiency virus SIVagm-infected African green monkeys
- PMID: 19109377
- PMCID: PMC2648264
- DOI: 10.1128/JVI.01841-08
Gag p27-specific B- and T-cell responses in Simian immunodeficiency virus SIVagm-infected African green monkeys
Abstract
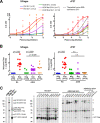
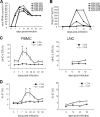
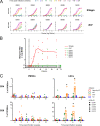
Nonpathogenic simian immunodeficiency virus SIVagm infection of African green monkeys (AGMs) is characterized by the absence of a robust antibody response against Gag p27. To determine if this is accompanied by a selective loss of T-cell responses to Gag p27, we studied CD4(+) and CD8(+) T-cell responses against Gag p27 and other SIVagm antigens in the peripheral blood and lymph nodes of acutely and chronically infected AGMs. Our data show that AGMs can mount a T-cell response against Gag p27, indicating that the absence of anti-p27 antibodies is not due to the absence of Gag p27-specific T cells.
Figures

Similar articles
-
Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts.J Virol. 2006 May;80(10):4858-67. doi: 10.1128/JVI.80.10.4858-4867.2006. J Virol. 2006. PMID: 16641277 Free PMC article.
-
CD8+ T lymphocytes of African green monkeys secrete an immunodeficiency virus-suppressing lymphokine.Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7207-11. doi: 10.1073/pnas.91.15.7207. Proc Natl Acad Sci U S A. 1994. PMID: 7913749 Free PMC article.
-
Experimental depletion of CD8+ cells in acutely SIVagm-infected African Green Monkeys results in increased viral replication.Retrovirology. 2010 May 11;7:42. doi: 10.1186/1742-4690-7-42. Retrovirology. 2010. PMID: 20459829 Free PMC article.
-
The role of the immune response during SIVagm infection of the African green monkey natural host.Front Biosci. 2004 Jan 1;9:550-64. doi: 10.2741/1219. Front Biosci. 2004. PMID: 14766390 Review.
-
SIVagm infection of its natural African green monkey host.Immunol Lett. 1996 Jun;51(1-2):53-8. doi: 10.1016/0165-2478(96)02555-2. Immunol Lett. 1996. PMID: 8811345 Review.
Cited by
-
Predominant envelope variable loop 2-specific and gp120-specific antibody-dependent cellular cytotoxicity antibody responses in acutely SIV-infected African green monkeys.Retrovirology. 2018 Mar 9;15(1):24. doi: 10.1186/s12977-018-0406-5. Retrovirology. 2018. PMID: 29523166 Free PMC article.
-
Early induction of polyfunctional simian immunodeficiency virus (SIV)-specific T lymphocytes and rapid disappearance of SIV from lymph nodes of sooty mangabeys during primary infection.J Immunol. 2011 May 1;186(9):5151-61. doi: 10.4049/jimmunol.1004110. Epub 2011 Mar 25. J Immunol. 2011. PMID: 21441446 Free PMC article.
-
Where the wild things are: pathogenesis of SIV infection in African nonhuman primate hosts.Curr HIV/AIDS Rep. 2010 Feb;7(1):28-36. doi: 10.1007/s11904-009-0034-8. Curr HIV/AIDS Rep. 2010. PMID: 20425055 Free PMC article. Review.
-
Distinct evolutionary pressures underlie diversity in simian immunodeficiency virus and human immunodeficiency virus lineages.J Virol. 2012 Dec;86(24):13217-31. doi: 10.1128/JVI.01862-12. Epub 2012 Oct 10. J Virol. 2012. PMID: 23055550 Free PMC article.
-
SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase.Microbes Infect. 2011 Jan;13(1):14-24. doi: 10.1016/j.micinf.2010.09.011. Epub 2010 Oct 15. Microbes Infect. 2011. PMID: 20951225 Free PMC article. Review.
References
-
- Allan, J. S., P. Kanda, R. C. Kennedy, E. K. Cobb, M. Anthony, and J. W. Eichberg. 1990. Isolation and characterization of simian immunodeficiency viruses from two subspecies of African green monkeys. AIDS Res. Hum. Retrovir. 6275-285. - PubMed
-
- Cumont, M. C., O. Diop, B. Vaslin, C. Elbim, L. Viollet, V. Monceaux, S. Lay, G. Silvestri, R. Le Grand, M. Muller-Trutwin, B. Hurtrel, and J. Estaquier. 2008. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Virol. 821175-1184. - PMC - PubMed
-
- Diop, O. M., A. Gueye, M. Dias-Tavares, C. Kornfeld, A. Faye, P. Ave, M. Huerre, S. Corbet, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2000. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 747538-7547. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

