Latent species C adenoviruses in human tonsil tissues
- PMID: 19109384
- PMCID: PMC2648257
- DOI: 10.1128/JVI.02392-08
Latent species C adenoviruses in human tonsil tissues
Abstract
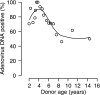
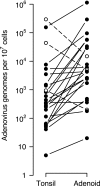
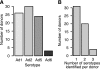
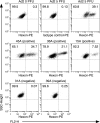
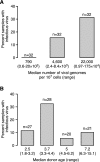
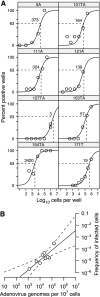
Although species C human adenoviruses establish persistent infections, the molecular details of this lifestyle remain poorly understood. We previously reported that adenovirus DNA is found in human mucosal T lymphocytes in a noninfectious form (C. T. Garnett, D. Erdman, W. Xu, and L. R. Gooding, J. Virol. 76:10608-10616, 2002). In this study, human tonsil and adenoid tissues were analyzed to determine the dynamics of infection, the rate of clearance of viral DNA, and the possibility of reactivation of virus from these tissues. The presence of viral DNA peaked at 4 years of age and declined thereafter. The average number of viral genomes declined with the age of the donor. The frequency of virus-bearing cells ranged from 3 x 10(-7) to 3.4 x 10(-4), while the amount of viral DNA per cell varied less, with an average of 280 copies per cell. All species C serotypes were represented in these tissues, although adenovirus type 6 was notably rare. Infectious virus was detected infrequently (13 of 94 of donors tested), even among donors with the highest levels of adenoviral DNA. Adenovirus transcripts were rarely detected in uncultured lymphocytes (2 of 12 donors) but appeared following stimulation and culture (11 of 13 donors). Viral DNA replication could be stimulated in most donor samples by lymphocyte stimulation in culture. New infectious virus was detected in 13 of 15 donors following in vitro stimulation. These data suggest that species C adenoviruses can establish latent infections in mucosal lymphocytes and that stimulation of these cells can cause viral reactivation resulting in RNA transcription, DNA replication, and infectious virus production.
Figures




References
-
- Avila, M. M., G. Carballal, H. Rovaletti, B. Ebekian, M. Cusminsky, and M. Weissenbacher. 1989. Viral etiology in acute lower respiratory infections in children from a closed community. Am. Rev. Respir. Dis. 140634-637. - PubMed
-
- Blanke, C., C. Clark, E. R. Broun, G. Tricot, I. Cunningham, K. Cornetta, A. Hedderman, and R. Hromas. 1995. Evolving pathogens in allogeneic bone marrow transplantation: increased fatal adenoviral infections. Am. J. Med. 99326-328. - PubMed
-
- Bowles, N. E., J. Ni, D. L. Kearney, M. Pauschinger, H. P. Schultheiss, R. McCarthy, J. Hare, J. T. Bricker, K. R. Bowles, and J. A. Towbin. 2003. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 42466-472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

