Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus
- PMID: 19109385
- PMCID: PMC2648275
- DOI: 10.1128/JVI.02309-08
Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus
Abstract
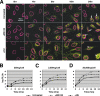
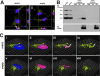
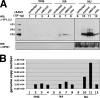
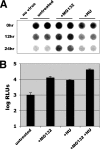
Adeno-associated virus (AAV) serotypes are being tailored for numerous therapeutic applications, but the parameters governing the subcellular fate of even the most highly characterized serotype, AAV2, remain unclear. To understand how cellular conditions control capsid trafficking, we have tracked the subcellular fate of recombinant AAV2 (rAAV2) vectors using confocal immunofluorescence, three-dimensional infection analysis, and subcellular fractionation. Here we report that a population of rAAV2 virions enters the nucleus and accumulates in the nucleolus after infection, whereas empty capsids are excluded from nuclear entry. Remarkably, after subcellular fractionation, virions accumulating in nucleoli were found to retain infectivity in secondary infections. Proteasome inhibitors known to enhance transduction were found to potentiate nucleolar accumulation. In contrast, hydroxyurea, which also increases transduction, mobilized virions into the nucleoplasm, suggesting that two separate pathways influence vector delivery in the nucleus. Using a small interfering RNA (siRNA) approach, we then evaluated whether nucleolar proteins B23/nucleophosmin and nucleolin, previously shown to interact with AAV2 capsids, affect trafficking and transduction efficiency. Similar to effects observed with proteasome inhibition, siRNA-mediated knockdown of nucleophosmin potentiated nucleolar accumulation and increased transduction 5- to 15-fold. Parallel to effects from hydroxyurea, knockdown of nucleolin mobilized capsids to the nucleoplasm and increased transduction 10- to 30-fold. Moreover, affecting both pathways simultaneously using drug and siRNA combinations was synergistic and increased transduction over 50-fold. Taken together, these results support the hypothesis that rAAV2 virions enter the nucleus intact and can be sequestered in the nucleolus in stable form. Mobilization from the nucleolus to nucleoplasmic sites likely permits uncoating and subsequent gene expression or genome degradation. In summary, with these studies we have refined our understanding of AAV2 trafficking dynamics and have identified cellular parameters that mobilize virions in the nucleus and significantly influence AAV infection.
Figures



References
-
- Atchison, R. W., B. C. Casto, and W. M. Hammon. 1966. Electron microscopy of adenovirus-associated virus (AAV) in cell cultures. Virology 29353-357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

