Inhibition of a large double-stranded DNA virus by MxA protein
- PMID: 19109387
- PMCID: PMC2643742
- DOI: 10.1128/JVI.00781-08
Inhibition of a large double-stranded DNA virus by MxA protein
Abstract
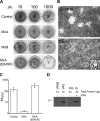
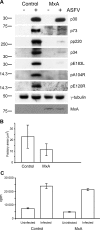
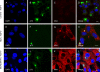
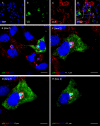
Increasing evidence points to the importance of the interferon (IFN) response in determining the host range and virulence of African swine fever virus (ASFV). Infection with attenuated strains of ASFV leads to the upregulation of genes controlled by IFN pathways, including myxovirus resistance (Mx) genes that are potent effectors of the antiviral state. Mx gene products are known to inhibit the replication of many negative-sense single-stranded RNA viruses, as well as double-stranded RNA viruses, positive-sense single-stranded RNA viruses, and the reverse-transcribing DNA virus hepatitis B virus. Here, we provide data that extend the known range of viruses inhibited by Mx to include the large double-stranded DNA viruses. Stably transfected Vero cells expressing human MxA protein did not support ASFV plaque formation, and virus replication in these cells was reduced 100-fold compared with that in control cells. In contrast, ASFV replication in cells expressing MxB protein or a mutant MxA protein was similar to that in control Vero cells. There was a drastic reduction in ASFV late protein synthesis in MxA-expressing cells, correlating with the results of previous work on the effect of IFN on viral replication. Strikingly, the inhibition of ASFV replication was linked to the recruitment of MxA protein to perinuclear viral assembly sites, where the protein surrounded the virus factories. Interactions between ASFV and MxA were similar to those seen between MxA and different RNA viruses, suggesting a common inhibitory mechanism.
Figures



References
-
- Afonso, C. L., L. Zsak, C. Carrillo, M. V. Borca, and D. L. Rock. 1998. African swine fever virus NL gene is not required for virus virulence. J. Gen. Virol. 792543-2547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

