Proteomic and immunologic analyses of brain tumor exosomes
- PMID: 19109410
- PMCID: PMC2669426
- DOI: 10.1096/fj.08-122184
Proteomic and immunologic analyses of brain tumor exosomes
Abstract
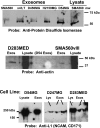
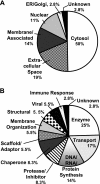
Brain tumors are horrific diseases with almost universally fatal outcomes; new therapeutics are desperately needed and will come from improved understandings of glioma biology. Exosomes are endosomally derived 30-100 nm membranous vesicles released from many cell types into the extracellular milieu; surprisingly, exosomes are virtually unstudied in neuro-oncology. These microvesicles were used as vaccines in other tumor settings, but their immunological significance is unevaluated in brain tumors. Our purpose here is to report the initial biochemical, proteomic, and immunological studies on murine brain tumor exosomes, following known procedures to isolate exosomes. Our findings show that these vesicles have biophysical characteristics and proteomic profiles similar to exosomes from other cell types but that brain tumor exosomes have unique features (e.g., very basic isoelectric points, expressing the mutated tumor antigen EGFRvIII and the putatively immunosuppressive cytokine TGF-beta). Administration of such exosomes into syngeneic animals produced both humoral and cellular immune responses in immunized hosts capable of rejecting subsequent tumor challenges but failed to prolong survival in established orthotopic models. Control animals received saline or cell lysate vaccines and showed no antitumor responses. Exosomes and microvesicles isolated from sera of patients with brain tumors also possess EGFR, EGFRvIII, and TGF-beta. We conclude that exosomes released from brain tumor cells are biochemically/biophysically like other exosomes and have immune-modulating properties. They can escape the blood-brain barrier, with potential systemic and distal signaling and immune consequences.
Figures


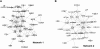


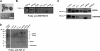
References
-
- Stupp R, Mason W P, van den Bent M J, Weller M, Fisher B, Taphoorn M J, Belanger K, Brandes A A, Marosi C, Bogdahn U, Curschmann J, Janzer R C, Ludwin S K, Gorlia T, Allgeier A, Lacombe D, Cairncross J G, Eisenhauer E, Mirimanoff R O. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. - PubMed
-
- Keller S, Sanderson M P, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. - PubMed
-
- Calzolari A, Raggi C, Deaglio S, Sposi N M, Stafsnes M, Fecchi K, Parolini I, Malavasi F, Peschle C, Sargiacomo M, Testa U. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. 2006;119:4486–4498. - PubMed
-
- Safaei R, Larson B J, Cheng T C, Gibson M A, Otani S, Naerdemann W, Howell S B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

