Autophagy provides nutrients but can lead to Chop-dependent induction of Bim to sensitize growth factor-deprived cells to apoptosis
- PMID: 19109422
- PMCID: PMC2642748
- DOI: 10.1091/mbc.e08-08-0829
Autophagy provides nutrients but can lead to Chop-dependent induction of Bim to sensitize growth factor-deprived cells to apoptosis
Abstract
Tissue homeostasis is controlled by the availability of growth factors, which sustain exogenous nutrient uptake and prevent apoptosis. Although autophagy can provide an alternate intracellular nutrient source to support essential basal metabolism of apoptosis-resistant growth factor-withdrawn cells, antiapoptotic Bcl-2 family proteins can suppress autophagy in some settings. Thus, the role of autophagy and interactions between autophagy and apoptosis in growth factor-withdrawn cells expressing Bcl-2 or Bcl-xL were unclear. Here we show autophagy was rapidly induced in hematopoietic cells upon growth factor withdrawal regardless of Bcl-2 or Bcl-xL expression and led to increased mitochondrial lipid oxidation. Deficiency in autophagy-essential gene expression, however, did not lead to metabolic catastrophe and rapid death of growth factor-deprived cells. Rather, inhibition of autophagy enhanced survival of cells with moderate Bcl-2 expression for greater than 1 wk, indicating that autophagy promoted cell death in this time frame. Cell death was not autophagic, but apoptotic, and relied on Chop-dependent induction of the proapoptotic Bcl-2 family protein Bim. Therefore, although ultimately important, autophagy-derived nutrients appear initially nonessential after growth factor withdrawal. Instead, autophagy promotes tissue homeostasis by sensitizing cells to apoptosis to ensure only the most apoptosis-resistant cells survive long-term using autophagy-derived nutrients when growth factor deprived.
Figures


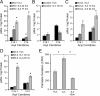



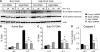

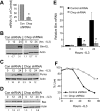

Comment in
- Mol Biol Cell. 20:1119.
References
-
- Abedin M. J., Wang D., McDonnell M. A., Lehmann U., Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–510. - PubMed
-
- Akdemir F., et al. Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development. 2006;133:1457–1465. - PubMed
-
- An J., Muoio D. M., Shiota M., Fujimoto Y., Cline G. W., Shulman G. I., Koves T. R., Stevens R., Millington D., Newgard C. B. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat. Med. 2004;10:268–274. - PubMed
-
- Bouillet P., Metcalf D., Huang D. C., Tarlinton D. M., Kay T. W., Kontgen F., Adams J. M., Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. - PubMed
-
- Colell A., et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

