Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms
- PMID: 19109430
- PMCID: PMC2642746
- DOI: 10.1091/mbc.e08-01-0065
Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms
Abstract
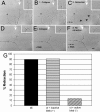
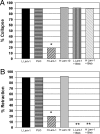
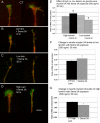
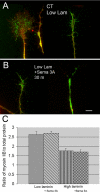
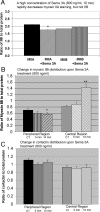
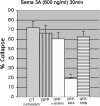
Growth cone responses to guidance cues provide the basis for neuronal pathfinding. Although many cues have been identified, less is known about how signals are translated into the cytoskeletal rearrangements that steer directional changes during pathfinding. Here we show that the response of dorsal root ganglion (DRG) neurons to Semaphorin 3A gradients can be divided into two steps: growth cone collapse and retraction. Collapse is inhibited by overexpression of myosin IIA or growth on high substrate-bound laminin-1. Inhibition of collapse also prevents retractions; however collapse can occur without retraction. Inhibition of myosin II activity with blebbistatin or by using neurons from myosin IIB knockouts inhibits retraction. Collapse is associated with movement of myosin IIA from the growth cone to the neurite. Myosin IIB redistributes from a broad distribution to the rear of the growth cone and neck of the connecting neurite. High substrate-bound laminin-1 prevents or reverses these changes. This suggests a model for the Sema 3A response that involves loss of growth cone myosin IIA to facilitate actin meshwork instability and collapse, followed by myosin IIB concentration at the rear of the cone and neck region where it associates with actin bundles to drive retraction.
Figures




References
-
- Ahmad F. J., Hughey J., Wittmann T., Hyman A., Greaser M., Baas P. W. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2000;5:276–280. - PubMed
-
- Allingham J. S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 2005;12:378–379. - PubMed
-
- Aizawa H., Wakatsuki S., Isii A., Moriyama K., Sasaki Y., Ohashi K., Sekine-Aizawa Y., Sehara-Fujisawa A., Mizuno K., Goshima Y., Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 2001;4:367–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

