Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons
- PMID: 19109503
- PMCID: PMC2859216
- DOI: 10.1523/JNEUROSCI.3398-08.2008
Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons
Abstract
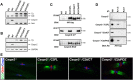
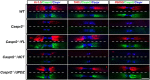
Clustering of Kv1 channels at the juxtaparanodal region (JXP) in myelinated axons depends on their association with the Caspr2/TAG-1 adhesion complex. The interaction between these channels and Caspr2 was suggested to depend on PDZ (PSD-95/Discs large/zona occludens-1) scaffolding proteins. Here, we show that at a subset of the JXP, PSD-93 colocalizes with Caspr2, K(+) channels and its related protein postsynaptic density protein-95 (PSD-95). The localization of PSD-93 and PSD-95 depends on the presence of Caspr2, as both scaffolding proteins failed to accumulate at the JXP in mice lacking either Caspr2 or TAG-1. In contrast, Caspr2 and K(+) channels still colocalized and associated in PSD-93, PSD-95 or double PSD-93/PSD-95 null mice. To directly evaluate the role of PDZ domain proteins in the function of Caspr2, we examined the ability of transgenic Caspr2 molecules lacking either their cytoplasmic domain (Caspr2dCT), or their PDZ-binding sequence (Caspr2dPDZ), to restore Kv1 channel clustering in Caspr2 null mice. We found that while Kv1 channels were distributed throughout internodes in nerves expressing Caspr2dCT, they were clustered at the JXP in axons expressing a full-length Caspr2 (Caspr2FL) or the Caspr2dPDZ transgene. Further proteomic analysis revealed that Caspr2 interacts with a distinct set of scaffolding proteins through its PDZ- and protein 4.1-binding sequences. These results demonstrate that while the molecular assembly of the JXP requires the cytoplasmic domain of Caspr2, its carboxy-terminal PDZ-binding motif is dispensable for Kv1 channel clustering. This mechanism is clearly distinct from the one operating at the axon initial segment, which requires PSD-93 for Kv1 channel clustering.
Figures

Similar articles
-
Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2.J Neurosci. 2008 May 28;28(22):5731-9. doi: 10.1523/JNEUROSCI.4431-07.2008. J Neurosci. 2008. PMID: 18509034 Free PMC article.
-
ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons.J Neurosci. 2010 Jan 20;30(3):1038-48. doi: 10.1523/JNEUROSCI.4661-09.2010. J Neurosci. 2010. PMID: 20089912 Free PMC article.
-
Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B.J Neurosci. 2010 Feb 17;30(7):2480-9. doi: 10.1523/JNEUROSCI.5225-09.2010. J Neurosci. 2010. PMID: 20164332 Free PMC article.
-
Clustering of neuronal potassium channels is independent of their interaction with PSD-95.J Cell Biol. 2002 Nov 25;159(4):663-72. doi: 10.1083/jcb.200206024. Epub 2002 Nov 18. J Cell Biol. 2002. PMID: 12438413 Free PMC article.
-
The Kv1-associated molecules TAG-1 and Caspr2 are selectively targeted to the axon initial segment in hippocampal neurons.J Cell Sci. 2017 Jul 1;130(13):2209-2220. doi: 10.1242/jcs.202267. Epub 2017 May 22. J Cell Sci. 2017. PMID: 28533267
Cited by
-
PAF-AH Catalytic Subunits Modulate the Wnt Pathway in Developing GABAergic Neurons.Front Cell Neurosci. 2010 May 28;4:19. doi: 10.3389/fncel.2010.00019. eCollection 2010. Front Cell Neurosci. 2010. PMID: 20725507 Free PMC article.
-
The axon initial segment in nervous system disease and injury.Eur J Neurosci. 2011 Nov;34(10):1609-19. doi: 10.1111/j.1460-9568.2011.07875.x. Eur J Neurosci. 2011. PMID: 22103418 Free PMC article. Review.
-
Clinical Character of CASPR2 Autoimmune Encephalitis: A Multiple Center Retrospective Study.Front Immunol. 2021 May 13;12:652864. doi: 10.3389/fimmu.2021.652864. eCollection 2021. Front Immunol. 2021. PMID: 34054814 Free PMC article.
-
White matter integrity in mice requires continuous myelin synthesis at the inner tongue.Nat Commun. 2022 Mar 4;13(1):1163. doi: 10.1038/s41467-022-28720-y. Nat Commun. 2022. PMID: 35246535 Free PMC article.
-
Protein 4.1B contributes to the organization of peripheral myelinated axons.PLoS One. 2011;6(9):e25043. doi: 10.1371/journal.pone.0025043. Epub 2011 Sep 26. PLoS One. 2011. PMID: 21966409 Free PMC article.
References
-
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K(+) channel clustering. J Neurosci Res. 1999;58:752–764. - PubMed
-
- Beer I, Barnea E, Ziv T, Admon A. Improving large-scale proteomics by clustering of mass spectrometry data. Proteomics. 2004;4:950–960. - PubMed
-
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology. 1996;35:851–865. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. - PMC - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires Neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
