Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia: a preclinical and phase 1 trial of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra) combined with etoposide
- PMID: 19109557
- PMCID: PMC2686135
- DOI: 10.1182/blood-2008-08-172726
Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia: a preclinical and phase 1 trial of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra) combined with etoposide
Abstract
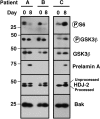
The farnesyltransferase inhibitor tipifarnib exhibits modest activity against acute myelogenous leukemia. To build on these results, we examined the effect of combining tipifarnib with other agents. Tipifarnib inhibited signaling downstream of the farnesylated small G protein Rheb and synergistically enhanced etoposide-induced antiproliferative effects in lymphohematopoietic cell lines and acute myelogenous leukemia isolates. We subsequently conducted a phase 1 trial of tipifarnib plus etoposide in adults over 70 years of age who were not candidates for conventional therapy. A total of 84 patients (median age, 77 years) received 224 cycles of oral tipifarnib (300-600 mg twice daily for 14 or 21 days) plus oral etoposide (100-200 mg daily on days 1-3 and 8-10). Dose-limiting toxicities occurred with 21-day tipifarnib. Complete remissions were achieved in 16 of 54 (30%) receiving 14-day tipifarnib versus 5 of 30 (17%) receiving 21-day tipifarnib. Complete remissions occurred in 50% of two 14-day tipifarnib cohorts: 3A (tipifarnib 600, etoposide 100) and 8A (tipifarnib 400, etoposide 200). In vivo, tipifarnib plus etoposide decreased ribosomal S6 protein phosphorylation and increased histone H2AX phosphorylation and apoptosis. Tipifarnib plus etoposide is a promising orally bioavailable regimen that warrants further evaluation in elderly adults who are not candidates for conventional induction chemotherapy. These clinical studies are registered at www.clinicaltrials.gov as #NCT00112853.
Figures
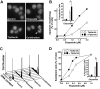
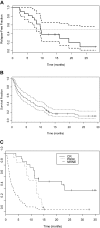
 ) for 84 adults treated with T + E. (C) OS for 21 patients achieving complete remission (CR, —), 13 patients achieving partial remission or hematologic improvement (PR/HI,
) for 84 adults treated with T + E. (C) OS for 21 patients achieving complete remission (CR, —), 13 patients achieving partial remission or hematologic improvement (PR/HI,  ), and 49 patients who did not achieve response (NR/NE, – –).
), and 49 patients who did not achieve response (NR/NE, – –).
Comment in
-
Tipifarnib and etoposide for older AML patients: from bench to bedside.Blood. 2009 May 14;113(20):4824-5. doi: 10.1182/blood-2009-02-201707. Blood. 2009. PMID: 19443668 No abstract available.
Similar articles
-
A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome.Cancer. 2011 Mar 15;117(6):1236-44. doi: 10.1002/cncr.25575. Epub 2010 Oct 19. Cancer. 2011. PMID: 20960519 Free PMC article. Clinical Trial.
-
Farnesyltransferase inhibitor tipifarnib inhibits Rheb prenylation and stabilizes Bax in acute myelogenous leukemia cells.Haematologica. 2014 Jan;99(1):60-9. doi: 10.3324/haematol.2013.087734. Epub 2013 Aug 30. Haematologica. 2014. PMID: 23996484 Free PMC article.
-
Multi-institutional phase 2 clinical and pharmacogenomic trial of tipifarnib plus etoposide for elderly adults with newly diagnosed acute myelogenous leukemia.Blood. 2012 Jan 5;119(1):55-63. doi: 10.1182/blood-2011-08-370825. Epub 2011 Oct 14. Blood. 2012. PMID: 22001391 Free PMC article. Clinical Trial.
-
Farnesyltransferase inhibition in hematologic malignancies: the clinical experience with tipifarnib.Clin Adv Hematol Oncol. 2008 Apr;6(4):303-10. Clin Adv Hematol Oncol. 2008. PMID: 18496498 Review.
-
Tipifarnib: farnesyl transferase inhibition at a crossroads.Expert Rev Anticancer Ther. 2006 Mar;6(3):313-9. doi: 10.1586/14737140.6.3.313. Expert Rev Anticancer Ther. 2006. PMID: 16503848 Review.
Cited by
-
The clinically relevant pharmacogenomic changes in acute myelogenous leukemia.Pharmacogenomics. 2012 Aug;13(11):1257-69. doi: 10.2217/pgs.12.102. Pharmacogenomics. 2012. PMID: 22920396 Free PMC article. Review.
-
Tipifarnib sensitizes cells to proteasome inhibition by blocking degradation of bortezomib-induced aggresomes.Blood. 2010 Dec 9;116(24):5285-8. doi: 10.1182/blood-2010-03-272393. Epub 2010 Sep 15. Blood. 2010. PMID: 20844234 Free PMC article.
-
Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers.Clin Cancer Res. 2010 Sep 15;16(18):4532-42. doi: 10.1158/1078-0432.CCR-10-0523. Epub 2010 Sep 7. Clin Cancer Res. 2010. PMID: 20823146 Free PMC article. Review.
-
H2AX: functional roles and potential applications.Chromosoma. 2009 Dec;118(6):683-92. doi: 10.1007/s00412-009-0234-4. Epub 2009 Aug 26. Chromosoma. 2009. PMID: 19707781 Free PMC article. Review.
-
The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients.Oncotarget. 2010 Jun;1(2):89-103. doi: 10.18632/oncotarget.114. Oncotarget. 2010. PMID: 20671809 Free PMC article. Review.
References
-
- Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. - PubMed
-
- Lancet JE, Willman CL, Bennett JM. Acute myelogenous leukemia and aging: clinical interactions. Hematol Oncol Clin North Am. 2000;14:251–267. - PubMed
-
- Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. - PubMed
-
- Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. - PubMed

