Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection
- PMID: 19109909
- PMCID: PMC2630178
- DOI: 10.1016/j.neuron.2008.11.029
Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection
Abstract
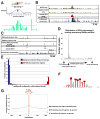
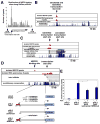
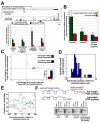
Although many transcription factors are known to control important aspects of neural development, the genome-wide programs that are directly regulated by these factors are not known. We have characterized the genetic program that is activated by MEF2, a key regulator of activity-dependent synapse development. These MEF2 target genes have diverse functions at synapses, revealing a broad role for MEF2 in synapse development. Several of the MEF2 targets are mutated in human neurological disorders including epilepsy and autism spectrum disorders, suggesting that these disorders may be caused by disruption of an activity-dependent gene program that controls synapse development. Our analyses also reveal that neuronal activity promotes alternative polyadenylation site usage at many of the MEF2 target genes, leading to the production of truncated mRNAs that may have different functions than their full-length counterparts. Taken together, these analyses suggest that the ubiquitously expressed transcription factor MEF2 regulates an intricate transcriptional program in neurons that controls synapse development.
Figures



References
-
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. - PubMed
-
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. - PubMed
-
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

