Roles of infection, inflammation, and the immune system in cholesterol gallstone formation
- PMID: 19109959
- PMCID: PMC2774219
- DOI: 10.1053/j.gastro.2008.12.031
Roles of infection, inflammation, and the immune system in cholesterol gallstone formation
Abstract
Cholesterol gallstone formation is a complex process mediated by genetic and environmental factors. Until recently, the role of the immune system in the pathogenesis of cholesterol gallstones was not considered a valid topic of research interest. This review collates and interprets an extensive body of basic literature, some of which is not customarily considered to be related to cholelithogenesis, describing the multiple facets of the immune system that appear to be involved in cholesterol cholelithogenesis. A thorough understanding of the immune interactions with biliary lipids and cholecystocytes should modify current views of the pathogenesis of cholesterol gallstones, promote further research on the pathways involved, and lead to novel diagnostic tools, treatments, and preventive measures.
Conflict of interest statement
The authors disclose no conflicts.
Figures


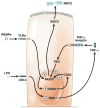

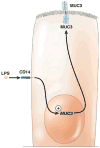
References
-
- Carey MC, Lamont JT. Cholesterol gallstone formation. 1. Physical-chemistry of bile and biliary lipid secretion. Prog Liver Dis. 1992;10:139–163. - PubMed
-
- Carey MC. Pathogenesis of gallstones. Am J Surg. 1993;165:410–419. - PubMed
-
- Paigen B, Carey MC. Gallstones. In: King RA, Rotter JI, Motulsky AG, editors. Genetic basis of common diseases. 2nd. New York, NY: Oxford University Press; 2002. pp. 298–335.
-
- Carey MC. Pathogenesis of gallstones. Recenti Prog Med. 1992;83:379–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

