Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a
- PMID: 19109969
- PMCID: PMC2710029
- DOI: 10.1016/j.yjmcc.2008.11.016
Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a
Abstract
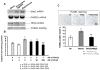
We have demonstrated that mesenchymal stem cells overexpressing the survival gene Akt can confer paracrine protection to ischemic myocytes both in vivo and in vitro through the release of secreted frizzled related protein 2 (Sfrp2). However, the mechanisms mediating these effects of Sfrp2 have not been fully elucidated. In this study, we studied rat cardiomyoblasts subjected to hypoxia reoxygenation (HR) injury to test the hypothesis that Sfrp2 exerts anti-apoptotic effect by antagonizing pro-apoptotic properties of specific Wnt ligands. We examined the effect of Wnt3a and Sfrp2 on HR-induced apoptosis. Wnt3a significantly increased cellular caspase activities and TUNEL staining in response to HR. Sfrp2 attenuated significantly Wnt3a-induced caspase activities in a concentration dependent fashion. Using a solid phase binding assay, our data demonstrates that Sfrp2 physically binds to Wnt3a. In addition, we observed that Sfrp2 dramatically inhibits the beta-catenin/TCF transcriptional activities induced by Wnt3a. Impressively, Dickkopf-1, a protein that binds to the Wnt coreceptor LRP, significantly inhibited the Wnt3a-activated caspase and transcriptional activities. Similarly, siRNA against beta-catenin markedly inhibited the Wnt3a-activated caspase activities. Consistent with this, significantly fewer TUNEL positive cells were observed in siRNA transfected cells than in control cells. Together, our data provide strong evidence to support the notion that Wnt3a is a canonical Wnt with pro-apoptotic action whose cellular activity is prevented by Sfrp2 through, at least in part, the direct binding of these molecules. These results can explain the in vivo protective effect of Sfrp2 and highlight its therapeutic potential for the ischemic heart.
Figures


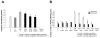

Similar articles
-
Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling.Biochem Biophys Res Commun. 2010 Sep 24;400(3):299-304. doi: 10.1016/j.bbrc.2010.08.043. Epub 2010 Aug 17. Biochem Biophys Res Commun. 2010. PMID: 20723538 Free PMC article.
-
IGF-1 enhances BMSC viability, migration, and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway.Stem Cell Res Ther. 2020 Jan 9;11(1):22. doi: 10.1186/s13287-019-1544-y. Stem Cell Res Ther. 2020. PMID: 31918758 Free PMC article.
-
SFRP2 regulates cardiomyogenic differentiation by inhibiting a positive transcriptional autofeedback loop of Wnt3a.Stem Cells. 2008 Jan;26(1):35-44. doi: 10.1634/stemcells.2007-0475. Epub 2007 Oct 4. Stem Cells. 2008. PMID: 17916803 Free PMC article.
-
Secreted frizzled-related protein 2 prevents pressure-overload-induced cardiac hypertrophy by targeting the Wnt/β-catenin pathway.Mol Cell Biochem. 2020 Sep;472(1-2):241-251. doi: 10.1007/s11010-020-03802-x. Epub 2020 Jul 6. Mol Cell Biochem. 2020. PMID: 32632611 Free PMC article.
-
sFRP2 activates Wnt/β-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling.Am J Physiol Cell Physiol. 2016 Nov 1;311(5):C710-C719. doi: 10.1152/ajpcell.00137.2016. Epub 2016 Sep 7. Am J Physiol Cell Physiol. 2016. PMID: 27605451 Free PMC article.
Cited by
-
Inverse Associations Between Circulating Secreted Frizzled Related Protein 2 (sFRP2) and Cardiometabolic Risk Factors.Front Cardiovasc Med. 2021 Oct 14;8:723205. doi: 10.3389/fcvm.2021.723205. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34722660 Free PMC article.
-
Mechanisms for progenitor cell-mediated repair for ischemic heart injury.Curr Stem Cell Res Ther. 2012 Jan;7(1):2-14. doi: 10.2174/157488812798483449. Curr Stem Cell Res Ther. 2012. PMID: 21466480 Free PMC article. Review.
-
SFRP2 enhances the osteogenic differentiation of apical papilla stem cells by antagonizing the canonical WNT pathway.Cell Mol Biol Lett. 2017 Aug 8;22:14. doi: 10.1186/s11658-017-0044-2. eCollection 2017. Cell Mol Biol Lett. 2017. PMID: 28794794 Free PMC article.
-
Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology.Circ Res. 2016 Jan 8;118(1):95-107. doi: 10.1161/CIRCRESAHA.115.305373. Circ Res. 2016. PMID: 26837742 Free PMC article. Review.
-
The role of Sfrp and DKK proteins in cardiomyocyte development.Physiol Rep. 2021 Feb;9(3):e14678. doi: 10.14814/phy2.14678. Physiol Rep. 2021. PMID: 33587322 Free PMC article. Review.
References
-
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt Prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003 Sep;9(9):1195–1201. - PubMed
-
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action acconts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005 Apr;11(4):367–368. - PubMed
-
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signaling from the structures of two Frizzled cysteine-rich domains. Nature. 2001 Jul 5;412(6842):86–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

