Expression of Muc19/Smgc gene products during murine sublingual gland development: cytodifferentiation and maturation of salivary mucous cells
- PMID: 19110483
- PMCID: PMC2664977
- DOI: 10.1369/jhc.2008.952853
Expression of Muc19/Smgc gene products during murine sublingual gland development: cytodifferentiation and maturation of salivary mucous cells
Abstract
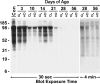
Muc19/Smgc expresses two splice variants, Smgc (submandibular gland protein C) and Muc19 (mucin 19), the latter a major exocrine product of differentiated murine sublingual mucous cells. Transcripts for Smgc were detected recently in neonatal sublingual glands, suggesting that SMGC proteins are expressed during initial salivary mucous cell cytodifferentiation. We therefore compared developmental expression of transcripts and translation products of Smgc and Muc19 in sublingual glands. We find abundant expression of SMGC within the initial terminal bulbs, with a subsequent decrease as Muc19 expression increases. During postnatal gland expansion, SMGC is found in presumptive newly formed acinar cells and then persists in putative acinar stem cells. Mucin levels increase 7-fold during the first 3 weeks of life, with little change in transcript levels, whereas between postnatal days 21 and 28, there is a 3-fold increase in Muc19 mRNA and heteronuclear RNA. Our collective results demonstrate the direct transition from SMGC to Muc19 expression during early mucous cell cytodifferentiation and further indicate developmentally regulated changes in Muc19/Smgc transcription, alternative splicing, and translation. These changes in Muc19/Smgc gene expression delineate multiple stages of salivary mucous cell cytodifferentiation and subsequent maturation during embryonic gland development through the first 4 weeks of postnatal life.
Figures


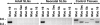


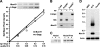

References
-
- Ball WD, Hand AR, Johnson AO (1988a) Secretory proteins as markers for cellular phenotypes in rat salivary glands. Dev Biol 125:265–279 - PubMed
-
- Ball WD, Hand AR, Moreira JE, Johnson AO (1988b) A secretory protein restricted to type I cells in neonatal rat submandibular glands. Dev Biol 129:464–475 - PubMed
-
- Ball WD, Redman RS (1984) Two independently regulated secretory systems within the acini of the submandibular gland of the perinatal rat. Eur J Cell Biol 33:112–122 - PubMed
-
- Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, Ann DK, et al. (2004) Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol 30:155–165 - PubMed
-
- Culp DJ, Latchney LR, Fallon M, Denny PA, Couwenhoven PC, Sally RI, Chuang S (2004) The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiol Genomics 19:303–318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

