Drug-virus interaction: effect of administration of recombinant adenoviruses on the pharmacokinetics of docetaxel in a rat model
- PMID: 19110543
- PMCID: PMC2765861
- DOI: 10.1038/cgt.2008.99
Drug-virus interaction: effect of administration of recombinant adenoviruses on the pharmacokinetics of docetaxel in a rat model
Abstract
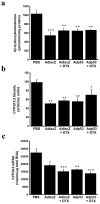
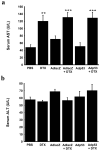
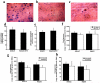
Modern cancer therapy combines recombinant viruses with traditional chemotherapeutic agents that are metabolized by hepatic cytochrome P450 3A4 (CYP3A4). A single dose of recombinant adenovirus (Ad) expressing beta-galactosidase (AdlacZ) significantly alters CYP3A2, the correlate of CYP3A4, in rats for 14 days. Recombinant adenovirus expressing human p53 (Adp53) also suppresses CYP3A2. Plasma clearance of docetaxel (DTX) in animals given AdlacZ (3.38+/-0.22 l h(-1) kg(-1)) was significantly lower than that of those given DTX alone (7.35+/-1.22 l h(-1) kg(-1), P<or=0.05). Area under the plasma concentration-time curve of DTX in rats given AdlacZ (2987.37+/-197.97 ng ml(-1) h(-1)) was significantly greater than those given drug alone (1496.14+/-281.62 ng ml(-1) h(-1), P<or=0.05). Both viruses prolonged DTX half-life (t(1/2)). Ad infection may cause significant variability in the pharmacokinetics and pharmacodynamics of anti-cancer agents and should be considered when designing therapeutic regimens for patients with viral infection and those enrolled in clinical trials using recombinant viruses.
Figures


Similar articles
-
Molecular and macromolecular alterations of recombinant adenoviral vectors do not resolve changes in hepatic drug metabolism during infection.Virol J. 2008 Sep 30;5:111. doi: 10.1186/1743-422X-5-111. Virol J. 2008. PMID: 18826641 Free PMC article.
-
Impact of transgene expression on drug metabolism following systemic adenoviral vector administration.J Gene Med. 2006 May;8(5):566-76. doi: 10.1002/jgm.884. J Gene Med. 2006. PMID: 16508909
-
Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel.Clin Pharmacol Ther. 2004 May;75(5):448-54. doi: 10.1016/j.clpt.2004.01.001. Clin Pharmacol Ther. 2004. PMID: 15116057 Clinical Trial.
-
Clinical pharmacokinetics of docetaxel.Clin Pharmacokinet. 1999 Feb;36(2):99-114. doi: 10.2165/00003088-199936020-00002. Clin Pharmacokinet. 1999. PMID: 10092957 Review.
-
Clinical pharmacokinetics of docetaxel : recent developments.Clin Pharmacokinet. 2006;45(3):235-52. doi: 10.2165/00003088-200645030-00002. Clin Pharmacokinet. 2006. PMID: 16509758 Review.
Cited by
-
Improving adenoviral vectors and strategies for prostate cancer gene therapy.Clinics (Sao Paulo). 2018 Aug 20;73(suppl 1):e476s. doi: 10.6061/clinics/2018/e476s. Clinics (Sao Paulo). 2018. PMID: 30133562 Free PMC article. Review.
-
Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity.Liver Int. 2012 Jan;32(1):8-20. doi: 10.1111/j.1478-3231.2011.02501.x. Epub 2011 Mar 14. Liver Int. 2012. PMID: 21745276 Free PMC article. Review.
-
Determination of 6258-70, a new semi-synthetic taxane, in rat plasma and tissues: Application to the pharmacokinetics and tissue distribution study.J Pharm Anal. 2016 Aug;6(4):219-225. doi: 10.1016/j.jpha.2016.02.003. Epub 2016 Mar 2. J Pharm Anal. 2016. PMID: 29403986 Free PMC article.
-
Immunization and Drug Metabolizing Enzymes: Focus on Hepatic Cytochrome P450 3A.Expert Rev Vaccines. 2021 May;20(5):623-634. doi: 10.1080/14760584.2021.1899818. Epub 2021 Mar 18. Expert Rev Vaccines. 2021. PMID: 33666138 Free PMC article.
-
Effect of isavuconazole on the pharmacokinetics of sunitinib and its mechanism.BMC Cancer. 2024 Sep 11;24(1):1131. doi: 10.1186/s12885-024-12904-4. BMC Cancer. 2024. PMID: 39261851 Free PMC article.
References
-
- Ritter T, Lehmann M, Volk HD. Improvements in gene therapy: averting the immune response to adenoviral vectors. BioDrugs. 2002;16(1):3–10. - PubMed
-
- Cao H, Koehler DR, Hu J. Adenoviral vectors for gene replacement therapy. Viral Immunol. 2004;17(3):327–333. - PubMed
-
- Vattemi E, Claudio PP. Adenoviral gene therapy in head and neck cancer. Drug News Perspect. 2006 Jul-Aug;19(6):329–337. - PubMed
-
- Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005 Sep;16(9):1016–1027. - PubMed
-
- Gurnani M, Lipari P, Dell J, Shi B, Nielsen LL. Adenovirus-mediated p53 gene therapy has greater efficacy when combined with chemotherapy against human head and neck, ovarian, prostate, and breast cancer. Cancer Chemother Pharmacol. 1999;44(2):143–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

